Figure 5.
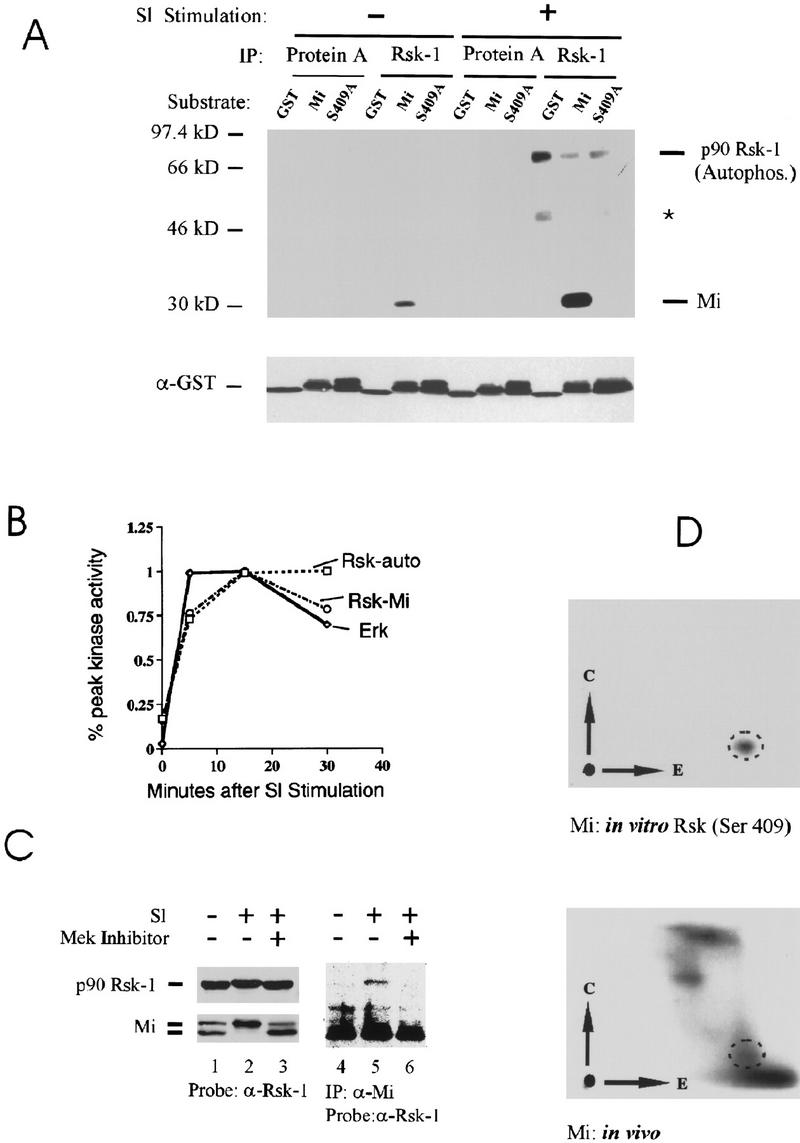
Involvement of Rsk-1 in phosphorylation of ser-409. (A) In vitro kinase assays were carried out using immunoprecipitated Rsk-1 (or protein A control) from unstimulated or Steel factor-stimulated (SI) melanoma cells. Kinase substrates consisted of GST (control), GST fusion to carboxy-terminal fragment of wild-type Mi (“Mi”), or GST fusion to carboxy-terminal fragment of Mi mutated at ser-409 to alanine (“S409A”). GST blot is shown at the bottom. Rsk-1 autophosphorylation is indicated and a weakly coprecipitating second Rsk substrate is indicated by asterisk. (B) Kinetics of Rsk and MAPK/ERK activation after SI stimulation. Kinase assays as described in A were quantitated by PhosphorImager and plotted as percent of maximal activity, indicating that Rsk activation lags ERK activation slightly. (C) Rsk-1 associates with Mi upon SI stimulation. Human melanoma cells were stimulated with SI (or unstimulated) in the presence or absence of MEK inhibitor drug (PD98059). Cell lysates were either probed directly for p90–Rsk-1 or Mi (lanes 1–3). MEK inhibition prevents a Steel-induced mobility shift in both Mi and a subtle mobility shift in Rsk-1. Mi was also immunoprecipitated from cell lysates followed by Western blotting for Rsk-1. Rsk-1 associated with Mi after Steel stimulation only if MAP kinase was not inhibited. (D) Two-dimensional phosphotryptic mapping indicates presence of phosphoserine 409 within endogenous cellular Mi. The two-dimensional mobility of phosphoserine 409 was determined by analyzing in vitro phosphorylated recombinant Mi (as in Fig. 5A). 32P-labeled melanoma cells were stimulated by SI and extracted; endogenous Mi was immunoprecipitated and subjected to tryptic digestion and two-dimensional analysis (simultaneously run with in vitro phosphorylated recombinant Mi). A spot was observed (dotted circles), which displays superimposable migration relative to the phosphoserine 409 spot generated by Rsk-1 phosphorylation of ser-409 in vitro.
