Figure 1.
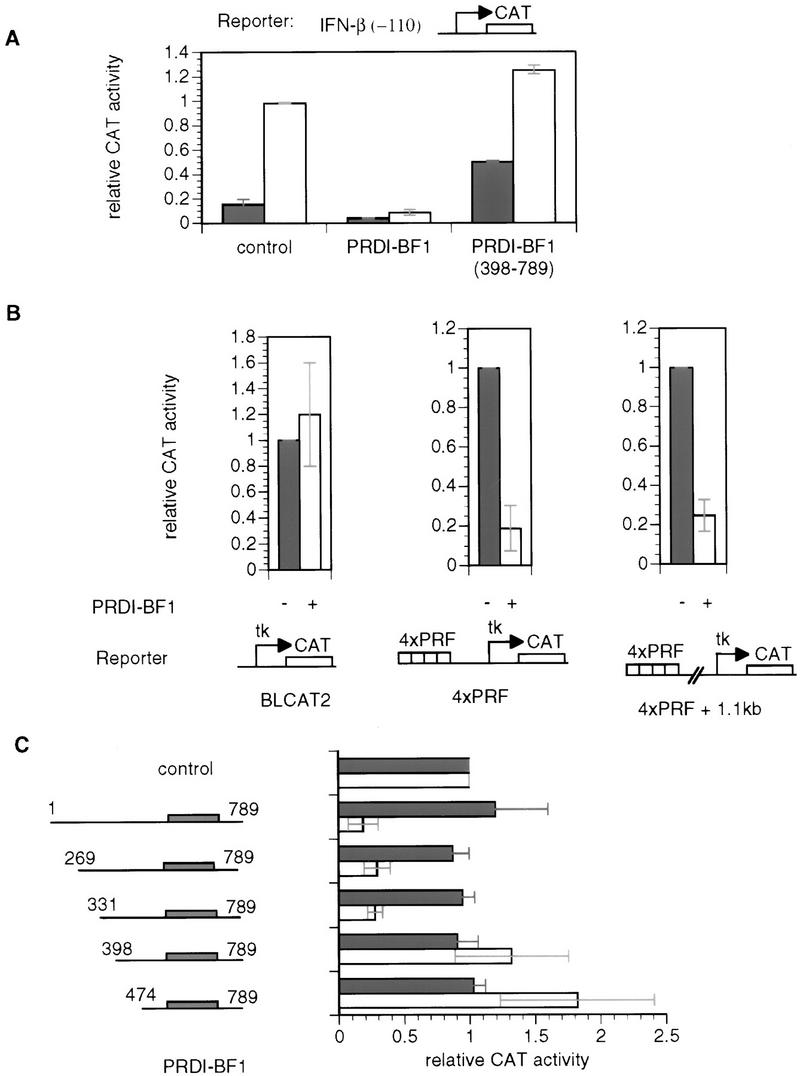
PRDI-BF1 is an active repressor. (A) PRDI-BF1 represses transcription from the natural IFN-β promoter, and this repression requires the amino terminus of the protein. The histogram shows the CAT activity produced in HeLa cells transfected with 5 μg of the reporter gene containing the −110 IFN-β promoter fused to the CAT gene, cotransfected with 1 μg of the indicated expression vector, 4 μg of the pcDNA3 vector, and 2 μg of pCMV–lacZ. The cells were either untreated (dark bars) or infected with Sendai virus (open bars) 24 hr after transfection, and harvested 16 hr later. (Control) pcDNA3 vector. CAT activities in this and the following experiments are normalized to the activity of the cotransfected pCMV–lacZ gene. (B) PRDI-BF1 represses transcription of the tk promoter when bound to sites located 1000 nucleotides from the start site of transcription. HeLa cells were transfected with 2 μg of pCMV–lacZ control plasmid, 6 μg of pXM, 3 μg of reporter, and 1 μg of effector pcDNA3 (−) or pcDNA3–PRDI-BF1 (+). The control reporter BLCAT2 contains a fragment (−109 to +55 bp) of the herpes simplex virus tk promoter driving the expression of the bacterial CAT gene. The 4×PRF reporter contains four copies of the PRF element inserted adjacent to the tk promoter. In the case of the 4×PRF + 1.1-kb reporter a 1.1-kb λDNA fragment was inserted between the tk promoter and the PRF elements. The data shown are representative of three independent assays. For each reporter, the CAT activity for PRDI-BF1 was normalized to that of the negative control pcDNA3. (C) Amino acids 331–398 of PRDI-BF1 functions as a transcriptional repression domain. The PRDI-BF1 sequences tested in each assay are illustrated at left. Horizontal lines represent PRDI-BF1 sequences of the full-length protein and various deletion mutants as illustrated. Shaded boxes denote the zinc finger DNA-binding domains of PRDI-BF1. PRDI-BF1 constructs (1 μg of each) were transfected into HeLa cells with the same control reporter and PRF-containing reporter (4×PRF) as in B. The data shown are representative of three independent assays, and the CAT activity for all PRDI-BF1 constructs was normalized by the CAT activity of the cells transfected with the control pcDNA3–Flag vector and the respective reporter BLCAT2 (dark bars) or 4×PRF–BLCAT2 (open bars).
