Figure 2.
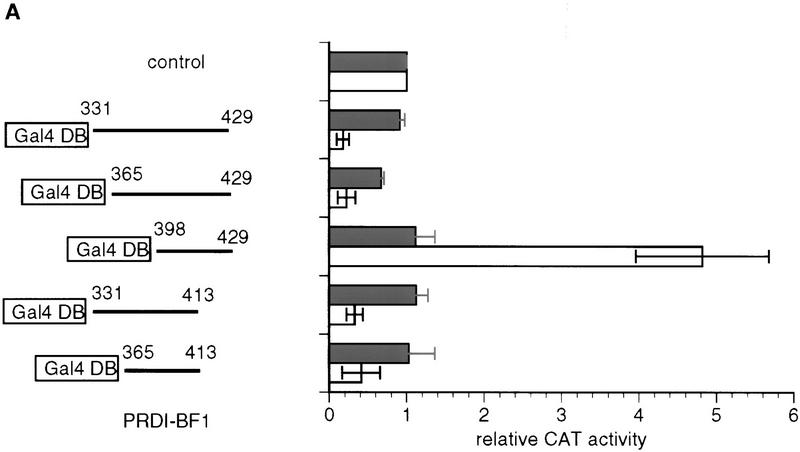
The PRDI-BF1 repression domain functions when fused to the Gal4-DNA-binding domain. (A) The ability of various Gal4–PRDI-BF1 fusion proteins to repress transcription was tested with a reporter that bears five Gal4 DNA-binding sites upstream of the tk promoter (G5BLCAT2). The BLCAT2 (see Fig. 1B) reporter was used as a control. Horizontal lines represent various PRDI-BF1 amino- and carboxy-terminal truncations cloned into the pBXG expression vector, which contains a DNA sequence encoding the Gal4 DNA-binding domain, Gal4(1–147) (boxed). PRDI-BF1 constructs (1 μg) were transfected into HeLa cells with either the control reporter BLCAT2 (dark bars) or G5BLCAT2 (open bars). The data shown are representative of three independent assays, and the CAT activities for all Gal4–PRDI-BF1 constructs were normalized by the CAT activity of the cells transfected with the control vector pECE and the respective reporter G5BLCAT2 or BLCAT2. (B) The sequence of a portion of the repression domain in PRDI-BF1. The sequence of the homologous region in Blimp-1 is also shown. The amino acid residues shared by the two proteins are listed between the two sequences. Two stretches of proline-rich regions, PRI and PRII, are indicated by brackets. (C) The proline-rich region of PRDI-BF1 is compared to similar region in WT1. Residues shared by PRDI-BF1 and WT1 protein are listed between the two sequences.