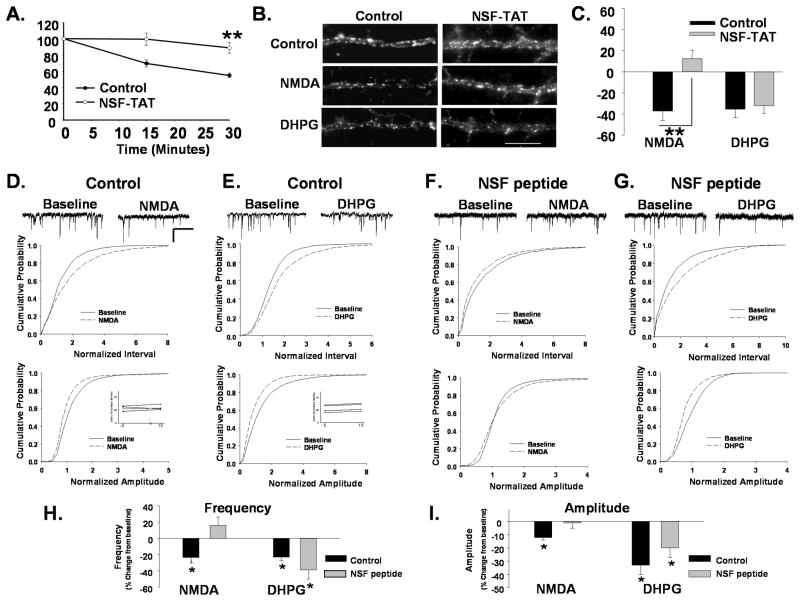
Figure 2. Disruption of AMPAR cycling blocks NMDAR but not mGluR-mediated receptor internalization.
A. Disruption of NSF blocks AMPAR cycling. Surface AMPARs in live neurons were immunolabeled, after which neurons were washed and incubated for different time intervals to allow constitutive receptor internalization to occur. Remaining surface AMPARs were labeled with 2° Ab following fixation. Loss of surface receptors over time provides a measure of constitutive AMPAR endocytosis. Graph depicts constitutive endocytosis of AMPARs over 30 minutes. The loss of surface AMPAR immunolabeling in control cells reveal the basal endocytosis associated with rapid receptor cycling. Neurons in which NSF-SNAP function has been disrupted with a TAT-tagged peptide (NSF-TAT) exhibit significantly reduced receptor endocytosis (n=5, **P< 0.01). B. Disruption of NSF prevents NMDAR- mediated AMPAR endocytosis. Representative images of surface GluA2 levels in dendrites of control peptide and NSF-TAT peptide-treated neurons with and without treatment with NMDA or DHPG to induce receptor internalization (Scale bar= 5 μm). C. Quantitation of experiments in B (n=7, **P<0.01) shows that the NSF-TAT peptide blocks the NMDA but not DHPG induced loss of surface GluA2s. D, E mEPSCs were recorded in cultured neurons during a 10 minute baseline period and 15 minutes after bath application of NMDA (D) or DHPG (E). Scale bar 500ms/20 pA. Cumulative distribution plots of mEPSC inter event intervals (top) and amplitude (bottom) demonstrate a reduction in the size and frequency of events with NMDA and DHPG treatment (NMDA n=6, Interval P< 0.05, Amplitude P< 0.05 KS test; DHPG, n=5, Interval P<0.001; Amplitude P<0.05 KS test). Inset shows series resistance at 5 minutes before (−5) and 15 minutes (15) after agonist application. F,G. The amplitude of mEPSCs was measured in neurons loaded with a peptide that disrupts NSF function (NSF peptide) through the recording pipette. mEPSC amplitude and inter event intervals were measured before and 15 minutes after a 1 minute application of NMDA (F) or DHPG (G). NMDA application caused no shift in the cumulative probability plots for mEPSC amplitudes and intervals in the presence of the NSF peptide (n=5, ns). DHPG application caused similar shifts the cumulative probability of mEPSC interval and amplitudes to controls when recorded in the presence of NSF peptide (n=5, Interval P<0.05; Amplitude P<0.01 KS test). The shift in amplitude was similar to controls but did not achieve significance. H. Graph showing the effects of various treatments on mean mEPSCs frequency (P< 0.05) demonstrates that disruption of NSF function blocks NMDA but not the DHPG induced depression in mEPSCs. I. Graph depicts changes in mEPSC amplitudes in response to agonist treatments (P< 0.05).