Figure 2.
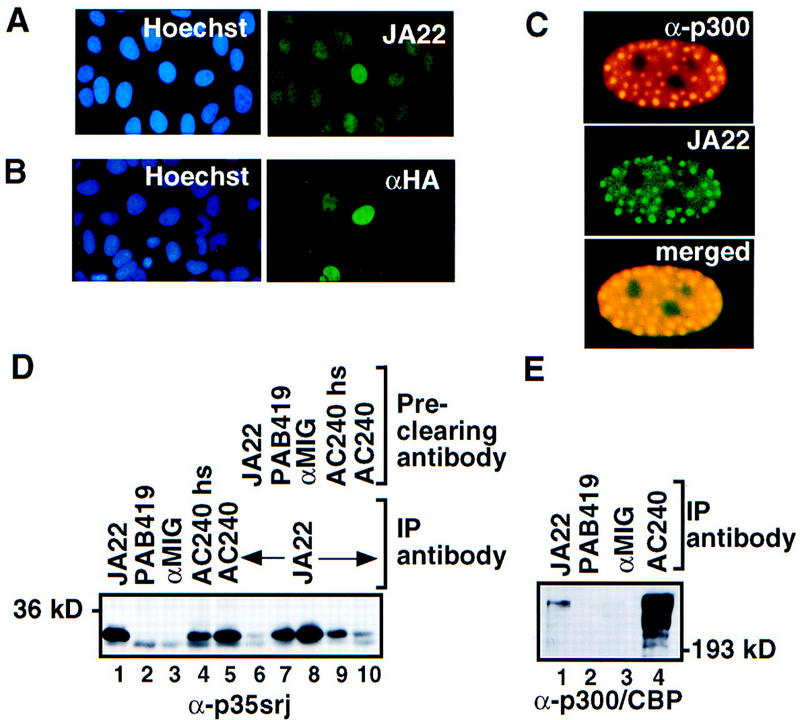
p35srj is a nuclear protein bound to p300/CBP. (A) Cellular localization of endogenous p35srj. U2-OS cells were stained with Hoechst to demonstrate cell nuclei, and with anti-p35srj monoclonal antibody JA22, to demonstrate endogenous p35srj. (B) U2-OS cells were transfected with HA–p35srj, cell nuclei stained with Hoechst, and immunostained with anti-HA monoclonal antibody. (C) Colocalization of endogenous p35srj with p300. U2-OS cells were transfected with HA–p300 and stained with anti-HA polyclonal antibody to reveal typical p300 dot-like structures characteristic of p300-overproducing cells. These cells were reacted simultaneously with anti-p35srj monoclonal antibody, JA22, to show the colocalization of endogenous p35srj in the p300 dots. The merged exposure confirms that the dots colocalize. (D) Efficiency of endogenous p35srj coimmunoprecipitation with anti-p300/CBP antibodies. Anti-p35srj Western blot of immunoprecipitates (IP) from U2-OS cell lysates. Immunoprecipitations were performed with monoclonal antibodies to p35srj (JA22, lane 1), control antibodies (PAB419 and rabbit anti-mouse IgG, αMIG, lanes 2,3), and antibody to p300/CBP (AC240) (lanes 4,5). The AC240hs immunoprecipitation (lane 4) was performed in 300 mm NaCl. The supernatants from the above immunoprecipitates were reprecipitated with JA22 (lanes 6–10) to assess the relative levels of free p35srj. (E) Anti-p300/CBP Western blot of immunoprecipitates (IP) from U2-OS cell lysates.
