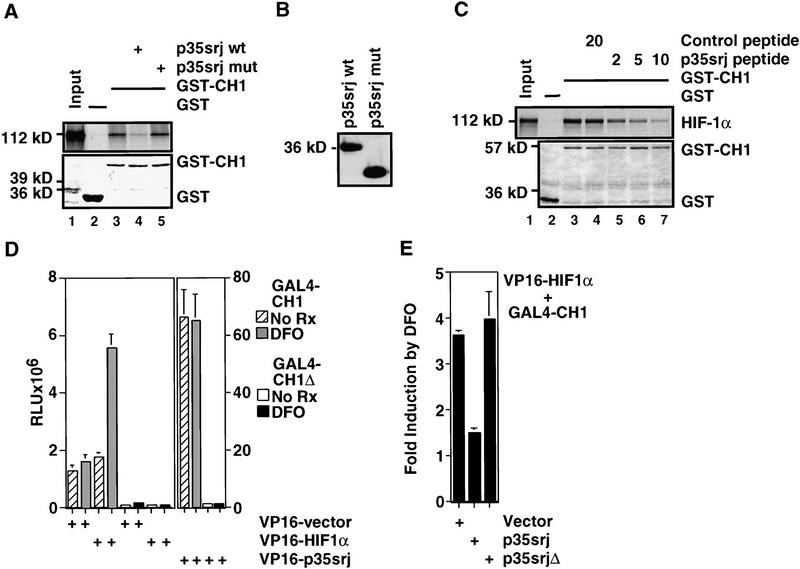
Figure 4.
p35srj competes with HIF-1α for binding to p300–CH1 in vitro and in vivo. (A) Effect of baculoviral p35srj on in vitro binding of HIF-1α to p300–CH1. (Top) The binding of 35S-labeled HIF-1α (lane 1) generated by in vitro translation to bacterially produced GST (lane 2) or GST–p300CH1 (lane 3) immobilized on glutathione–Sepharose beads was tested. The binding of HIF-1α to GST–p300CH1 was also tested in the presence of baculovirally expressed wild-type (lane 4) or mutant p35srj (expressing p35srj residues 1–160) as control (lane 5). (Bottom) Coomassie stain of the gel demonstrating relative amounts of the GST and GST–p300CH1 proteins. (B) Anti-p35srj Western blot of SF21 cell lysates used in A. (C) Effect of p35srj 224–255 peptide on in vitro binding of HIF-1α to p300–CH1. (Top) The binding of HIF-1α to GST–p300CH1 was tested in the presence of increasing concentrations of the wild-type peptide (lanes 5–8) and compared to either a control, irrelevant peptide (lane 4) or no peptide (lane 3).Amounts of peptide used are shown in micrograms. Twenty percent of the HIF-1α input was loaded in lane 1. (Bottom) Coomassie stain of the gel demonstrating relative amounts of the GST and GST–p300CH1 proteins. (D) Effect of DFO on a mammalian two-hybrid interaction between GAL4–CH1 and VP16–HIF1α 723–826 (left) or VP16–p35srj (right). Hep3B cells were cotransfected with the indicated GAL4 and VP16 fusion plasmids (40 ng each), 3× GAL4–luc reporter (100 ng), and CMV–lacZ (100 ng). GAL4–CH1 contains p300 residues 300–528. GAL4–CH1Δ lacks p300 residues 346–410, and served as a control. Results are presented as relative luciferase units (RLU, mean of three independent experiments ± s.e.m.). (E) Effect of p35srj on the two-hybrid interaction between GAL4–p300CH1 and VP16–HIF1α. Hep3B cells were cotransfected with VP16HIF1α and GAL4–CH1 (40 ng each), and either vector control or p35srj expression plasmids (80 ng each), and CMV–lacZ (100 ng). p35srjΔ lacks residues 215–270. Results (mean of three independent experiments ± s.e.m.) are presented as fold induction of luciferase activity by DFO. A fold induction of 1 represents absence of induction.