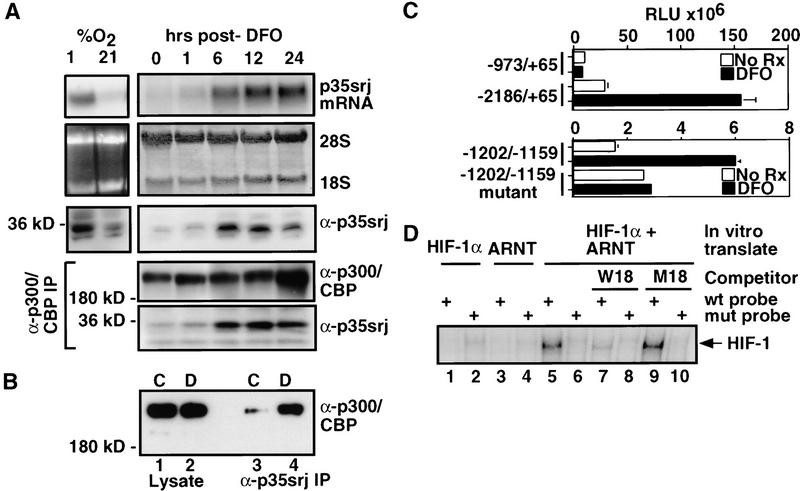
Figure 6.
p35srj is induced by deferoxamine and hypoxia. (A) Hep3B cells were grown to confluence and exposed to either 1 or 21% O2 for 6 hr or to 100 μm DFO for the indicated periods of time. Total RNA from these cells was Northern blotted and probed for p35srj (top). 28S and 18S rRNA are shown as loading controls. Whole-cell lysates (50 μg per lane) from these cells were Western blotted to detect p35srj, using JA22 mAb (α-p35srj). Cell lysates were also immunoprecipitated with anti-p300/CBP mAb, AC240 (α-p300/CBP IP), and Western blotted with anti-p300/CBP monoclonal antibodies (α-p300/CBP) and anti-p35srj monoclonal antibody JA22 (α-p35srj, bottom). (B) Hep3B cells were grown to confluence and exposed to 100 μm DFO for 5 hr. Cell lysates from control (C) cells or DFO treated (D) cells were immunoprecipitated with anti-p35srj monoclonal antibody JA22 (lanes 3 and 4, respectively), and Western blotted with anti-p300/CBP monoclonal antibodies. One-fortieth of the total cell lysate used for each immunoprecipitation was loaded in lanes 1 and 2, respectively. (C) Effect of DFO on the p35srj promoter. Hep3B cells were transfected with the indicated human p35srj promoter–luciferase construct (300 ng), and CMV–lacZ (100 ng). The −1202/−1159 wild-type reporter plasmid contains the sequence 5′-gtgtgcgcgtggtgccatacgggacgtgcagctacgtgcccacc, whereas the −1202/−1159 mutant contains the mutated sequence 5′-gtgtgcgaaaggtgccatacgggaaaagcagctaaaagcccacc, cloned upstream of TK–luciferase. The HIF-1 consensus sequences in the wild-type are underlined. Results are presented as RLU (mean of three independent experiments ± s.e.m.). (D) The p35srj promoter binds HIF-1 in vitro. A gel-shift assay using 32P-labeled double-stranded wild-type and mutant oligonucleotide probes, derived from the p35srj promoter (see B), was performed. The probes were incubated with HIF-1α, ARNT, or both proteins, which were generated by in vitro translation. Oligonucleotides, W18 and M18, are wild-type and mutant competitors for HIF-1 binding, and were introduced at 100 fold molar excess.