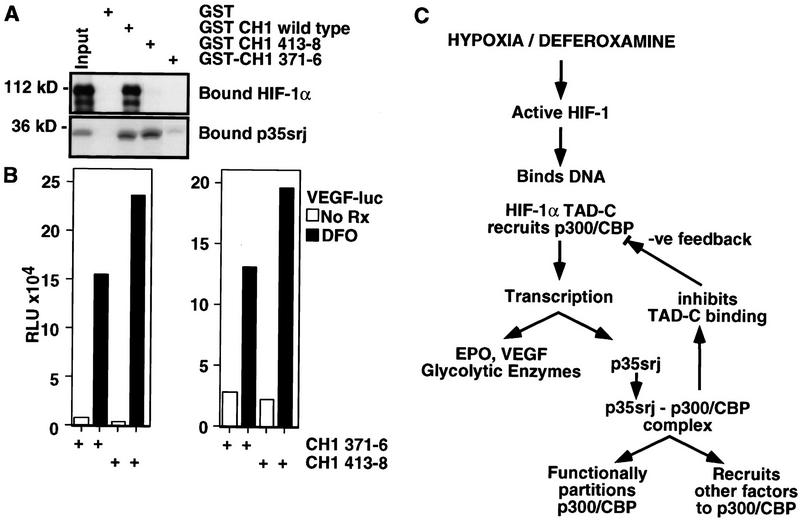
Figure 7.
Sequestering p35srj enhances HIF-1 transactivation. (A) Effect of mutations in GST p300–CH1 on binding to HIF-1α and to p35srj. GST–CH1 wild type includes p300 amino acids 300–528. In GST–CH1–371–6, and CH1–413–8, the indicated residues were replaced with a NAAIRS sequence. The relative efficiency of binding of in vitro translated HIF-1α or p35srj to the wild-type and mutant GST–CH1 peptides was assayed. (B) Effect of mutant CH1 peptides on activation of VEGF promoter by deferoxamine. The mutants are the NAAIRS-substituted derivatives of CH1 (p300 residues 300–528) described in A. Hep3B cells were transiently cotransfected with a VEGF–luc reporter (40 ng), the indicated HA-tagged CH1 expression plasmids (300 ng each) and CMV–lacZ (100 ng). Results are presented as fold induction of the VEGF reporter by DFO relative to the uninduced activity. Two independent experiments are shown. The cells in the experiment represented at left were analyzed at a higher density than those at right. (C) Model: Putative roles of p35srj in HIF-1 transactivation.