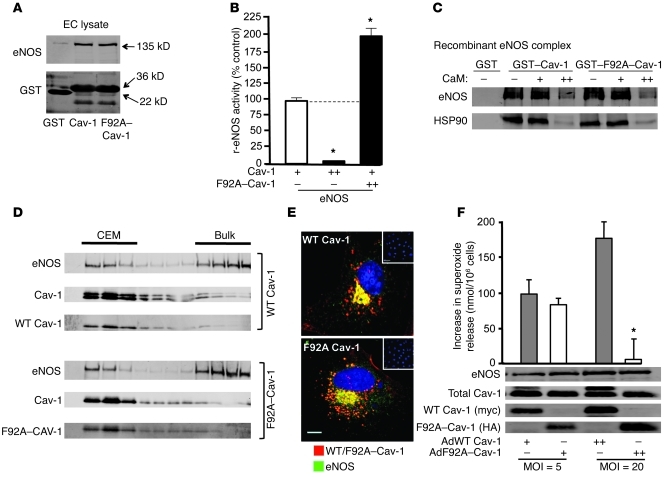
Figure 3. F92A–Cav-1 interacts with eNOS and prevents Cav-1 from inhibiting eNOS.
(A) BAEC lysates were incubated with GSH-coupled beads coated with GST alone, GST–Cav-1 (aa 62–101), or GST–F92A–Cav-1 (aa 62–101), washed, and eNOS binding determined by immunoblotting. Top panels show interaction of eNOS with Cav-1 domain and the bottom panel documents a similar amount of GST protein input. Performed in triplicate. (B) Recombinant eNOS activity assay. Purified eNOS was incubated with limiting amounts of GST–Cav-1–coated beads (+) or supplemented with excess soluble GST–Cav-1 (++) or excess soluble GST–F92A–Cav-1 (++) and NOS activity quantified. n = 5 in triplicate. *P < 0.05. (C) CaM/HSP90-dependent displacement of eNOS binding to GST–Cav-1 and GST–F92A–Cav-1. eNOS (2 μg) and HSP90 (4 μg) were incubated with beads in the absence (–) or presence of 0.01 (+) or 1 (++) μM of CaM. Experiments were performed in triplicate; typical data shown. (D) F92A–Cav-1 does not affect its cosedimentation or trafficking (E) with eNOS. WT Cav-1 (myc, top) or F92A–Cav-1 (HA, bottom) localization was examined by confocal microscopy by using anti-myc/HA (red) or anti-eNOS (green). Nuclei were visualized with DAPI (blue). Inset depicts lack of myc or HA staining in noninfected cells. n = 5 individual cells. Scale bar: 7 μm. (F) Decreased superoxide release in BAECs infected with F92A–Cav-1. BAECs were infected with Ad-WT and Ad-F92A–Cav-1 for 48 hours, and superoxide formation was evaluated by cytochrome c reduction. Western blots show protein expression for each condition. (n = 7–10; *P < 0.05).