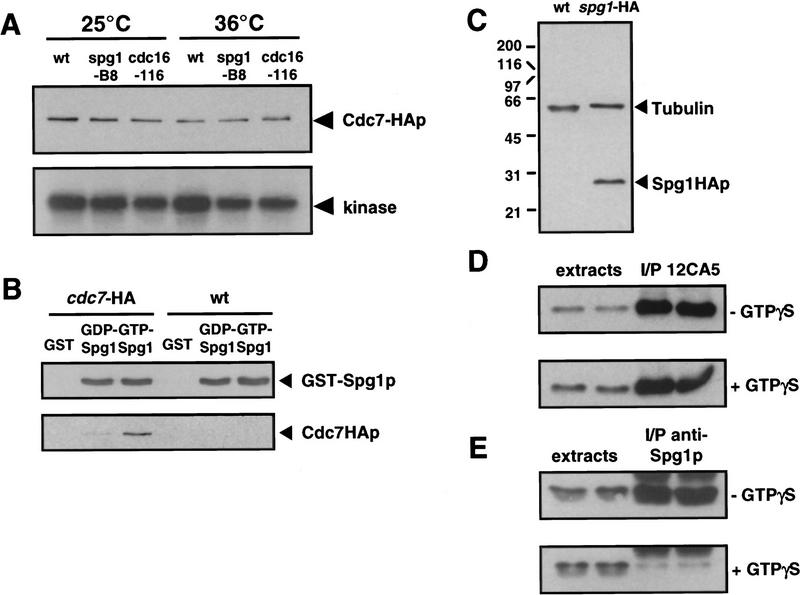
Figure 2.
Cdc7p binds to the GTP-bound form of Spg1p, which is not recognized by the anti-Spg1p antiserum (SuSu1). (A) Cells carrying the cdc7–HA allele in a wild-type, spg1-B8, or cdc16-116 background were grown in YE medium at 25°C. Half the culture was shifted to 36°C for 4 hr. Protein extracts were prepared and Cdc7p was immunoprecipitated with the anti-Cdc7p antiserum. The kinase activity associated with the precipitate was assayed using myelin basic protein as in vitro substrate (performed at 25°C or 36°C, respectively). The amount of protein in the immunoprecipitates was analyzed by Western blotting with mAb 12CA5. (B) GST, the GDP-, or the GTP-bound form of GST–Spg1p were mixed with protein extracts prepared from wild-type (972) or cdc7–HA cells. The complexes precipitated with glutathione–agarose were Western blotted and probed with anti-Spg1p antiserum (SuSu1) and mAb 12CA5. (C) Total proteins were prepared from wild-type (972) and spg1–HA cells. The Western blot was probed with a mixture of TAT-1 (loading control) and mAb 12CA5. (D) Proteins were prepared from spg1–HA cells in the absence (top) or presence (bottom) of GTPγS, and Spg1–HAp was immunoprecipitated with mAb 12CA5. The soluble extracts and the immunoprecipitates were analyzed by Western blotting with mAb 12CA5. Note that equal amounts of Spg1–HAp are immunoprecipitated in the presence or absence of GTPγS. (E) Same as in D, except that proteins were prepared from wild-type cells (972), and the anti-Spg1p antibody (SuSu1) was used for immunoprecipitation and Western blotting. Note that much less protein is precipitated by this serum in the presence of GTPγS, suggesting that the antibody recognizes preferentially GDP–Spg1p.