Figure 2.
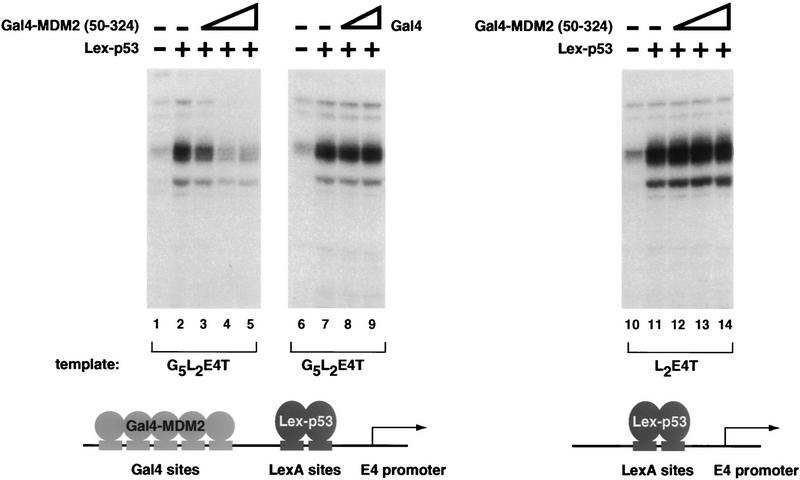
Restoring the ability of MDM2(50–324) to be targeted to a promoter enables it to inhibit p53 activation domain-dependent transactivation. To restore the ability of MDM2(50–324) to be recruited to the promoter, amino acids 50–324 of human MDM2 were fused to the Gal4 DNA-binding domain. The ability of G4–MDM2(50–324) to inhibit transcription from a template containing five Gal4 sites upstream of two LexA sites and the E4 promoter (G5L2E4T) was assayed by primer extension using the transcription system described in Fig. 1. p53 activation domain-dependent stimulation was acheived using a protein containing the LexA DNA-binding domain (amino acids 1–202) fused to two tandem copies of p53 amino acids 1–42 (Lex–p53). Lane 1 contains no added activator; lanes 2–5 have 30 ng of Lex–p53. The inhibitory properties of G4–MDM2(50–324) were tested by adding 20, 60, and 200 ng of this protein to the transcription reactions (lanes 3–5). The Gal4 DNA-binding domain alone was also tested for its ability to repress transcription from the G5L2E4T template. Lane 6 contains no added activator; lanes 7–9 contain 30 ng of Lex–p53. Lanes 8 and 9 also contain 20 and 60 ng of Gal4, respectively. The dependence of G4–MDM2(50–324) inhibition on the presence of the Gal4 sites was tested by performing the same series of reactions described for lanes 1–5 but using a template containing only two LexA sites upstream of the E4 promoter (L2E4T) (lanes 10–14).