Figure 4.
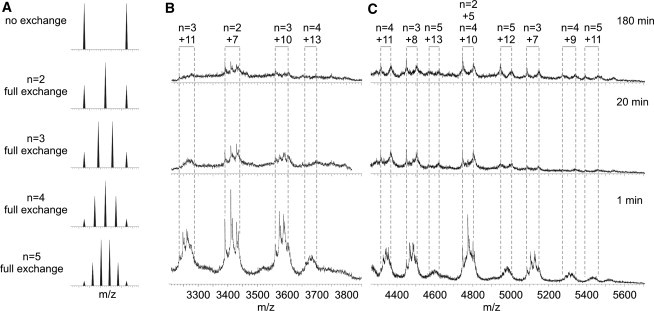
β2m oligomer dynamics change as a function of assembly time. 14N- and 15N-labeled β2m were incubated separately under WL fibril-forming conditions (0.4 mg mL−1 based on monomer; 100 mM ammonium acetate, pH 3.6). The two samples were mixed in an equimolar ratio at various time points and ESI-MS spectra acquired within 1 min of mixing. (A) Predicted m/z spectra for dimer, trimer, tetramer, and pentamer, assuming full 14N/15N-labeled subunit exchange has occurred. If subunit exchange with the bulk protein pool is rapid then, in the case of the dimer (n = 2), a 1:2:1 ratio of 14N/14N:14N/15N:15N/15N will be observed. If the exchange is slow or does not occur, then a 1:1 ratio of 14N/14N:15N/15N will be observed. (B) ESI-MS spectra (m/z 3200–3800) at t = 1, 20, and 180 min. The dimer and the more highly charged trimer (+10, +11) and tetramer (+13) ions exchange rapidly throughout the time course, but are barely populated by 180 min. (C) ESI-MS spectra (m/z 4300–5600) at t = 1, 20, and 180 min. The lower charge-state ions of the trimer (+7, +8), the tetramer (+9, +10, +11) and all the pentamer ions exchange rapidly at t = 1 min but display slow exchange at 180 min, indicating less-dynamic quaternary structures.
