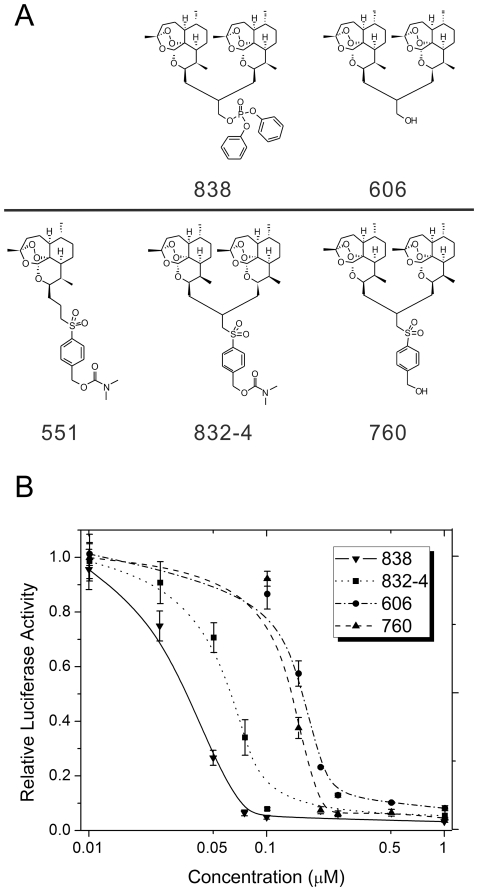
Figure 2. Chemical structure and anti-CMV activity of the two most potent artemisinin-derived dimers and their corresponding parent compounds.
A: Chemical structures of the two most potent artemisinin-derived dimers 838 and 832-4 and their corresponding parent alcohols 606 and 760. Monomer 551 is depicted on the left of dimer 832-4. The sulfone benzylic dimethyl carbamate unit is the same in monomer 551 as in dimer 832-4. Therefore, the enhanced anti-CMV activity of the dimer 832-4 over monomer 551 is not due to any difference in the sulfone carbamate portion of the molecule. B: Detailed anti-CMV activity of dimers 832-4 and 838, and their respective parent compounds 760 and 606. CMV-infected HFF were treated with dimers 832-4 and 838 at the indicated concentrations. 72 hpi luciferase activity was determined in the lysates of infected cells. Luciferase values represent the mean and SD of data derived from at least four independent experiments performed in triplicates. The curve fitting toolbox, Matlab software (v7.5), Mathworks (Natick, MA) was used to determine EC50 values using a four-parameter logistic regression.