Figure 1.
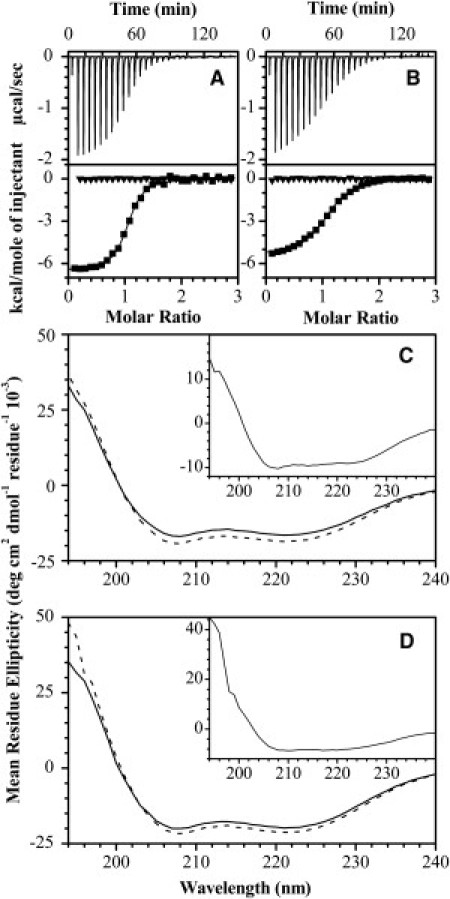
Isothermal titration calorimetry and circular dichroism spectroscopy of MBP-derived peptides interacting with Ca2+-activated CaM. A 100 μM protein solution (20 mM HEPES-KOH, pH 7.4, 100 mM KCl, 5 mM CaCl2) was titrated with 1 mM solution of α1-peptide (A) and α3-peptide (B) at 30°C (-▪-). The Ca2+-dependency of the binding was assessed in the same reaction protocol, but now in the Ca2+-depleted buffer (20 mM HEPES-KOH, pH 7.4, 100 mM KCl, 8 mM EDTA, 2 mM EGTA) (-▾-). The data were fitted to the software Origin's one-set-of-sites model (OriginLab, Northampton, MA). All parameters are summarized in Table S1. The CD spectra of 131 μM and 176 μM Ca2+-CaM in the absence (solid lines) or presence (dashed lines) of equimolar amounts of α1- or α3-peptide are shown in panels C and D, respectively. (Insets) Difference spectra of Ca2+-CaM complexes with peptides minus Ca2+-CaM.
