Figure 2.
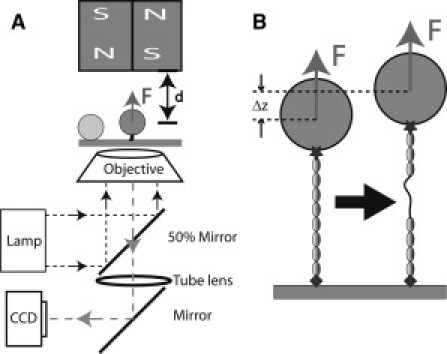
(A) Schematic representation of the magnetic-tweezers setup. The protein is tethered between a paramagnetic bead and the coverslip surface. Force is applied to the bead by a pair of permanent magnets above the sample, and changing the distance, d, between the magnets and the bead controls the magnitude of the force. Beads are illuminated by light through the objective and imaged by charge-coupled device. A bead stuck on the surface is used as a reference to eliminate drift in three dimensions. (B) Schematic representation of the unfolding of a domain in the tethered protein. Domain unfolding leads to an abrupt increase in the height of the bead, Δz. The resulting change in the bead diffraction pattern is used to measure Δz at a resolution of ∼2 nm.
