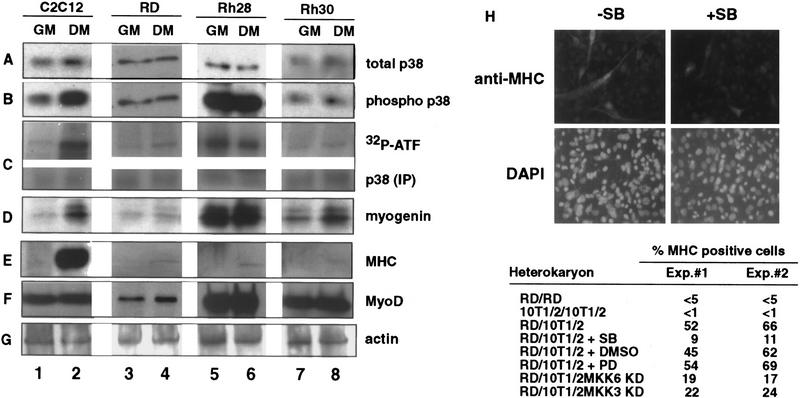
Figure 2.
Human RMS cell lines express p38 MAPK but do not modulate its activity under differentiation conditions. The levels of p38 MAPK (A) and phosphorylated p38 MAPK (B) were determined by immunoblotting with the appropriate antibodies (see Materials and Methods) in total lysates of mouse C2C12 (lanes 1,2) and three human RMS cell lines: RD (lanes 3,4), Rh28 (lanes 5,6), and Rh30 (lanes 7,8). (GM) Growth medium; (DM) differentiation medium. (C) p38 MAPK activity was measured by an immunocomplex kinase assay using GST-ATF2 fusion protein as a substrate. The levels of myogenin (D), MHC (E), MyoD (F), and actin (G) were also determined by immunoblotting with the appropriate antibodies. The formation of heterokaryons of RD and 10T/12 fibroblasts (H) was performed as described previously (Tapscott et al. 1993). The heterokaryons were transferred into DM with (+SB) or without (−SB) SB203580 (5 μm) to inhibit p38 MAPK. DMSO and PD98059 were also used as negative controls. After 36 hr in DM, heterokaryons were fixed and stained for the expression of MHC (top) and for the visualization of nuclei with DAPI (bottom). The different pattern of nuclear staining–punctate for mouse nuclei in 10T1/2 cells and uniform staining for human nuclei in RD cells—indicates the formation of heterokaryons. The number of MHC-positive heterokaryons/field was scored and the results of two experiments are summarized. In the case of MKK3KD and MKK6KD overexpression, these dominant-negative forms were expressed by transient transfection in 10T1/2 prior the fusion with RD. A GFP-encoding plasmid was cotransfected to localize successfully transfected cells. The number of GFP/MHC-positive heterokaryons/field was scored and the results of two experiments are summarized.