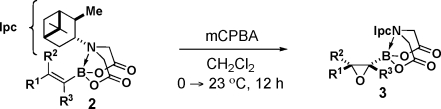
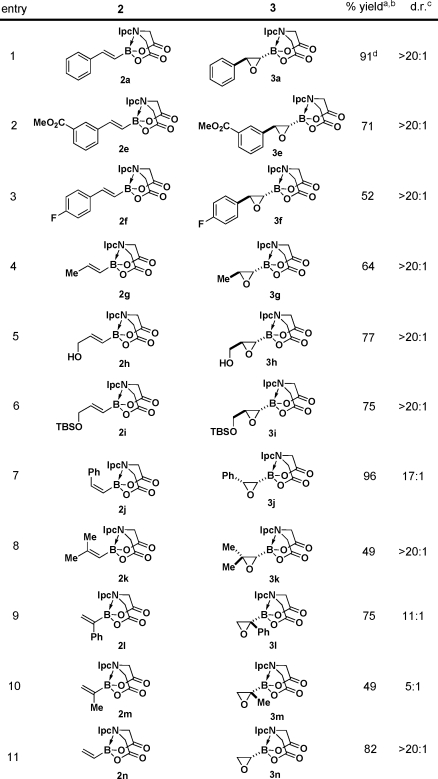
Table 2. Highly Diastereoselective Epoxidations of a Wide Range of Alkenyl PIDA Boronates.
Isolated yields after silica gel chromatography.
The stereochemistries of epoxides 3a, 3g, and 3n were all determined unambiguously via single-crystal X-ray analysis. The remaining product configurations were assigned by analogy.
Diastereomeric ratios determined via 500 MHz 1H NMR analysis of the unpurified reaction mixtures.
Conducted on a 15 mmol scale and isolated by crystallization.