Table 1.
Optimization of the Annulation of (Sa)-1 with Benzaldehyde and tert-Butyl Carbamate
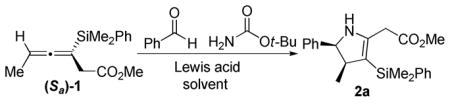 | |||||
|---|---|---|---|---|---|
| entry | Lewis acid | time (h) | solvent | yield of 2aa | drc |
| 1 | TMSOTf | 48 | DCM | 9% | >20:1 |
| 2 | TMSOTf | 48 | EtCN | 41% | >20:1 |
| 3 | TiCl4 | 48 | DCM | <5% | >20:1 |
| 4 | TiCl4 | 48 | EtCN | 33% | >20:1 |
| 5 | TfOH | 48 | DCM | 44% | >20:1 |
| 6 | TfOH | 48 | EtCN | 47% | >20:1 |
| 7 | BF3 · OEt2 | 72 | DCM | 14% | >20:1 |
| 8 | BF3 · OEt2 | 72 | MeCN | 56% | >20:1 |
| 9 | BF3 · OEt2 | 72 | EtCN | 67% | >20:1 |
Isolated yields after purification over silica gel. All reactions run with 1.2 equiv Lewis acid at −78 to −40 °C except for entry 8 run at −40 °C.
Diastereomeric ratios were determined by 1H NMR analysis on crude material.
