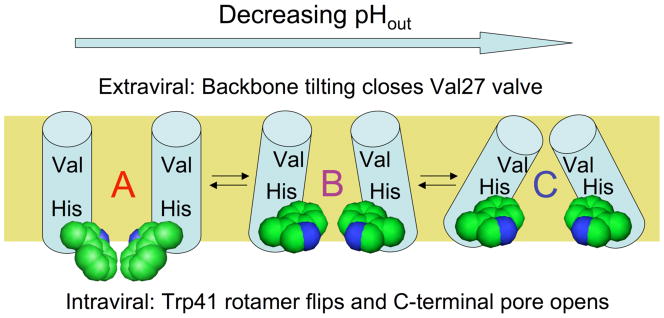
Figure 1.
Proposed trajectory of structural transitions in the M2 transmembrane domain as a function of extraviral pH. The “A” conformation is based on an NMR structure in micelles at high pH (31), the intermediate “B” conformation is proposed from molecular dynamics experiments on M2 protonation states (34) and intermediate pH crystal forms of the tetramer (33), while the “C” conformation is based on a crystal structure of M2 at low pH (32). The helix bundle is depicted side-on with the front two helices removed. The proposed conformational changes upon lowering N-terminal pH involve closure of the N-terminal Val27 valve by increasing helix tilt at the top of the bundle, while at the C-terminal end of the bundle the Trp41 sidechains (space filling representation) undergo a rotamer shift, then separate as a result of increased helix tilt. These changes control exposure of the proton-binding His37 sidechains near the middle of the bundle to N-terminal and C-terminal pore waters.