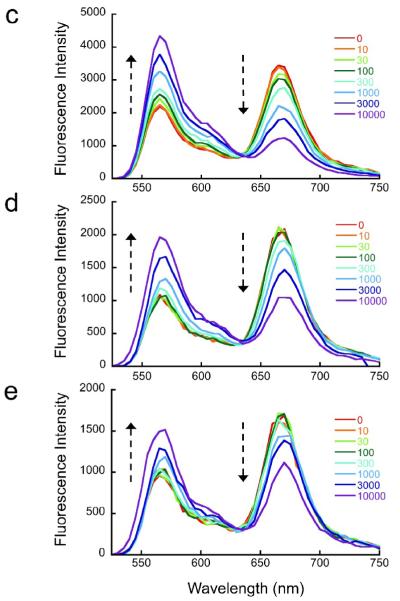
Fig. 1. Experimental systems for stopped flow FRET analyses.
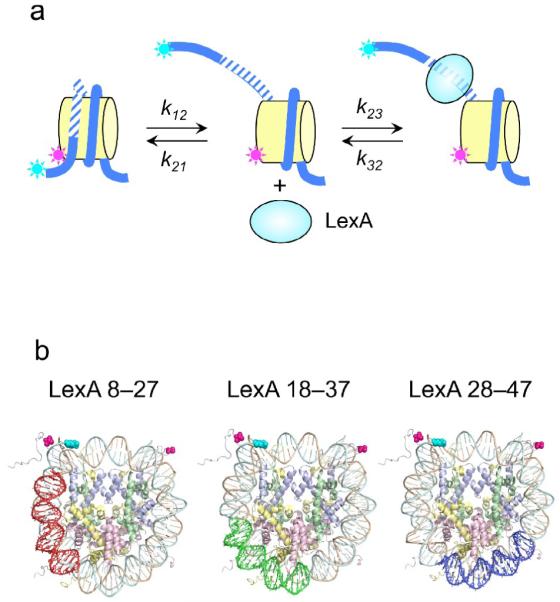
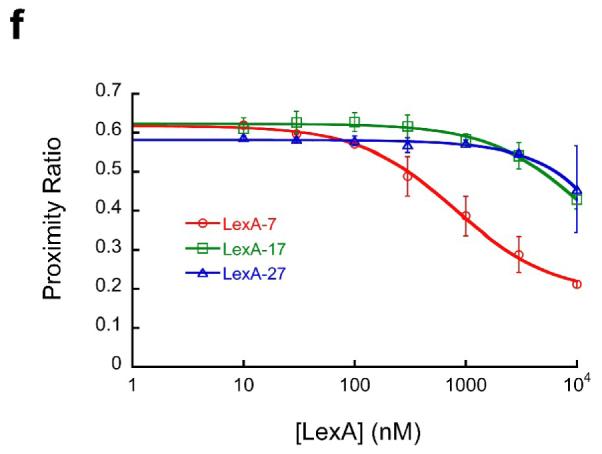
(a) Schematic illustration of experimental design. Nucleosomes are constructed having a fluorescent donor (Cy3, cyan) attached to one end of the DNA, and a fluorescent acceptor (Cy5, magenta) attached nearby on the histone protein core, to a unique engineered cysteine residue on H3 V35C C110A. A second, symmetry-related copy is located too far away to yield significant FRET. Spontaneous partial DNA unwrapping (middle panel) exposes a buried DNA target site (hatched) for the site specific DNA binding protein LexA. When LexA protein is rapidly added at sufficiently high concentration (200 nM or greater; ref.16), the rate of binding (k23 times the concentration of LexA) is much greater than the rewrapping rate (k21), trapping nucleosomes in the unwrapped state as these occur. In this regime, the observed relaxation rate equals the intrinsic unwrapping rate itself, k12. (b) Structural illustrations of three systems having the same FRET dye pair but moving the LexA target site inward toward the middle of the nucleosome in 10 bp steps. The actual basepairs comprising the target site are indicated. The overall nucleosome length is 147 bp, with the center at bp 74. (c–e) Normalized steady state fluorescence emission spectra from LexA titrations of the three systems in panel (b), respectively. Addition of increasing concentrations of LexA lead to increased Cy3 emission (at ~570 nm) and decreased Cy5 emission (at ~670 nm), implying decreased FRET efficiency. (f) Proximity ratios calculated from the data of panels (c-e); the mean and standard deviation (n=3) are indicated. The curves represent least-squares fits to a non-cooperative binding isotherm; the apparent affinities for the LexA 18–37 and 28–47 systems are too low to determine accurately given practical limits on the concentrations of LexA.