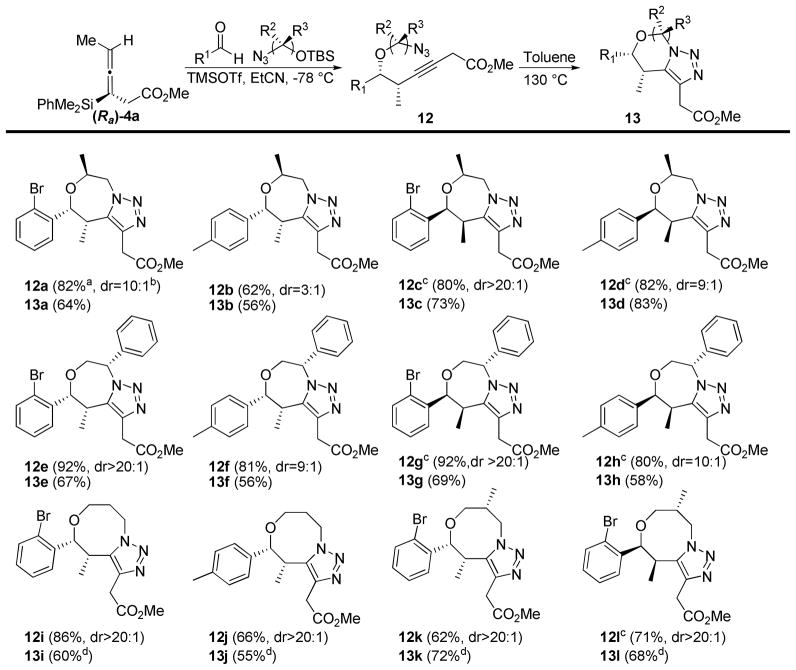
Scheme 4.
Propargylations and dipolar cycloadditions with substituted silyl ethers
a All yields refer to isolated products after purification over silica gel. b All diastereomeric ratios were determined by 1H NMR analysis on crude material.
c The (Sa) enantiomer of the allenylsilane 1 was used for the propargylation reaction. dCycloaddition reaction run in chlorobenzene at 150 ºC.