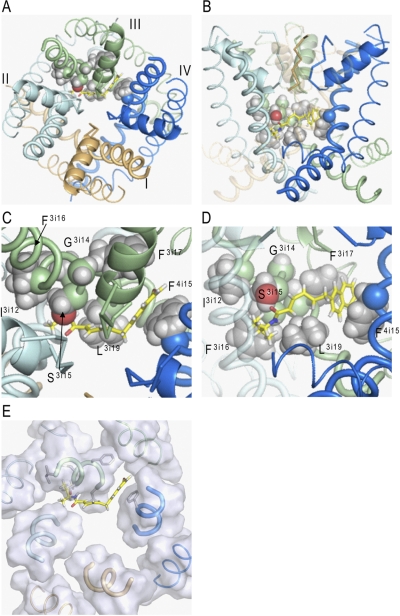
Fig. 4.
BTG 502 in the pore domain of BgNav1-1. Repeats I, II, III, and IV are colored brown, gray, green, and blue, respectively. S5s, P-helices, and S6s are shown as thin, medium-sized, and thick helices, respectively. The ascending limbs are shown as thin C-α tracings. The ligand (rendered by sticks with yellow carbons) wraps around IIIS6 and exposes its termini into the II/III and III/IV domain interfaces. Shown are the top (A) and side (B) views and their zoomed images (C and D). E, top view of the pore domain in which parts of the transmembrane helices at the level of the bound BTG 502 are shown by transparent surfaces. In the proposed binding mode, BTG 502 would partially obstruct the ion permeation by the hydrophobic linker exposed into the pore (A, C, and E). The isopropyl group of BTG 502 contacts residues Ile3i12 and Phe3i16, which do not face the pore, whereas the amide oxygen accepts an H-bond from Ser3i15 (C). Leu3i19 is below the hydrophobic linker (D). The naphthalene ring of BTG 502 protrudes in the interface between domains III and IV, where it is flanked by Phe3i17 and Phe4i15 and forms π-stacking interactions with these residues (C and D).