Abstract
In addition to increasing cGMP, the soluble guanylyl cyclase (sGC) activator 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) can elevate intracellular cAMP levels. This response was assumed to be as a result of cGMP-dependent inhibition of cAMP phosphodiesterases; however, in this study, we show that YC-1-induced cAMP production in the rat pancreatic beta cell line INS-1E occurs independent of its function as a sGC activator and independent of its ability to inhibit phosphodiesterases. This YC-1-induced cAMP increase is dependent upon soluble adenylyl cyclase and not on transmembrane adenylyl cyclase activity. We previously showed that soluble adenylyl cyclase-generated cAMP can lead to extracellular signal-regulated kinase activation and that YC-1-stimulated cAMP production also stimulates extracellular signal-regulated kinase. Although YC-1 has been used as a tool for investigating sGC and cGMP-mediated pathways, this study reveals cGMP-independent pharmacological actions of this compound.
Introduction
The derivative of benzylindazole 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) was the first of a class of compounds identified to directly activate soluble guanylyl cyclase (sGC). Work on purified sGC (Mülsch et al., 1997) revealed that YC-1 stimulates sGC in a heme-dependent and nitric oxide (NO)-independent manner (Ko et al., 1994; Wu et al., 1995; Friebe et al., 1996). YC-1 can sensitize the enzyme for NO activation in vitro (Friebe et al., 1996), in human platelets (Teng et al., 1997), and in smooth muscle (Galle et al., 1999), and it has been shown to enhance extracellular signal-regulated kinase (ERK) and cAMP response element-binding protein phosphorylation in amygdala and hippocampus through a NO-cGMP-protein kinase G pathway (Chien et al., 2003, 2008). Thus, YC-1 has been a valuable reagent for exploring sGC/cGMP-dependent signaling pathways in biological systems.
YC-1 has other reported effects as well. It inhibited the activity of cyclic nucleotide-catabolizing phosphodiesterases (PDEs) in aortic extracts, specifically isoforms 1 to 5 (Galle et al., 1999). This effect would augment the elevation of cGMP levels due to the stimulation of sGC. Other reported activities of YC-1, which were found to be independent of its effects on sGC activity, included stimulating NO production in endothelial cells (Wohlfart et al., 1999), protecting optic nerves (Garthwaite et al., 2002), inhibiting voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells (Park et al., 2010), inhibiting respiratory burst and degranulation in human neutrophils (Hwang et al., 2003), and inhibiting proliferation of mesangial cells through p38 mitogen-activated protein kinase (MAPK) activation (Chiang et al., 2005).
YC-1 also has been reported to elevate levels of the distinct second messenger cAMP. In platelets (Ko et al., 1994) and human neutrophils (Hwang et al., 2003), YC-1 increases cAMP levels (and protein kinase A activity). Such effects were postulated to be mediated via the inhibition of a putative cGMP-inhibited cAMP PDE (Ko et al., 1994).
During the course of our studies exploring the role of cAMP in beta cells of the pancreas, we found that YC-1 also elevated cAMP, independent of any effect on cGMP, in the beta cell-like insulinoma cell line INS-1E. Beta cells produce cAMP when exposed to high concentrations of glucose (Charles et al., 1973; Grill and Cerasi, 1973; Rutter, 2001; Tian et al., 2011), and this response can be studied in INS-1E cells (Ramos et al., 2008). We previously demonstrated that the bicarbonate-, calcium-, and ATP-regulated soluble adenylyl cyclase (sAC) is at least partially responsible for glucose-induced cAMP generation in INS-1E cells and that glucose-induced ERK phosphorylation is exclusively dependent upon sAC-generated cAMP (Ramos et al., 2008). We now demonstrate that YC-1 elevates cAMP accumulation in INS-1E cells independent of its effects on sGC or cAMP-catabolizing PDEs and dependent upon sAC. Thus, although it has long been appreciated that cAMP levels can be affected during YC-1 treatment, we now demonstrate that such effects are cGMP independent and reveal a unique, unknown direct target of YC-1 actions.
Materials and Methods
Reagents.
INS-1E cells were a gift from Claus Wollheim (University Medical Center, Geneva, Switzerland). RPMI 1640 medium, l-glutamine, and HEPES were acquired from Cellgro (Manassas, VA). Dimethyl sulfoxide, β-mercaptoethanol, sodium pyruvate, and glucose were obtained from Sigma-Aldrich (St. Louis, MO), and fetal bovine serum was obtained from Gemini Bio-Products (West Sacramento, CA). Phospho-ERK and total ERK rabbit polyclonal antibodies were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-linked anti-mouse and anti-rabbit antibodies and SuperSignal West Pico chemiluminescent substrate were obtained from Thermo Fisher Scientific (Waltham, MA). The anti-sAC monoclonal antibody R21 was developed in our laboratory and is directed against amino acids 203 to 216 of human sAC.
The following reagents were used in this study: YC-1, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 8-bromo-4H-2,5-dioxa-3,9b-diaza-cyclopenta[a]naphthalen-1-one (NS2028), and 2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-(4-morpholinyl)-4,6-pyrimidinediamine (BAY 41-8543) (Cayman Chemical, Ann Arbor, MI). S-Nitroso-N-acetylpenicillamine (SNAP) and 2′,5′-dideoxyadenosine (2′,5′-ddAdo) were obtained from EMD Chemicals (Gibbstown, NJ), and 3-isobutyl-1-methylxanthine (IBMX) was obtained from Sigma-Aldrich. The sAC-specific inhibitor 2-(1H-benzimidazol-2-ylthio)-2-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide propanoic acid (KH7) was synthesized by ChemDiv, Inc. (San Diego, CA) and by the Abby and Howard P. Milstein Synthetic Chemistry Core Facility of Weill Cornell Medical College.
Cell Culture.
INS-1E cells (passage 150–175) were cultured as described previously (Asfari et al., 1992). The cells were passaged every 3 days and cultured under 5% CO2 in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol.
Adenylyl Cyclase Assays and cAMP Determinations.
In vitro adenylyl cyclase (AC) activity was measured on purified recombinant rat truncated sAC (sACt) protein as described previously (Buck et al., 1999; Chen et al., 2000; Litvin et al., 2003). For in vivo cAMP accumulation assays, 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. Two days later, cells were incubated in 2.5 mM glucose Krebs-Ringer solution (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1% bovine serum albumin for 1 to 2 h before the start of the assay. At time 0, media was replaced with Krebs-Ringer solution containing 2.5 mM glucose or 16 mM glucose in the presence of 500 μM IBMX and the different inhibitors or vehicle controls as specified in the figure legends. We found no difference in accumulated cAMP levels whether IBMX was preincubated for 10 min or added simultaneously with glucose or the various drugs (data not shown). Cells were incubated for the indicated time at 37°C, followed by aspiration of media, and the cells were lysed with 200 μl of 0.1 M HCl per well. Intracellular cAMP content was determined using Correlate-EIA cAMP Direct Assay (Enzo Life Sciences, Farmingdale, NY).
For assays of in vitro transcription/translation products, rat full-length sAC (sACfl) and sACt cDNAs were expressed (Buck et al., 1999). In vitro transcription/translation was performed using the TNT Quick Coupled Transcription/Translation System (Promega, Madison, WI) according to the manufacturer's instructions. Synthesis of sACfl and sACt proteins was confirmed by Western blot with the sAC monoclonal antibody R21. cAMP production was measured with 15 μl of in vitro transcription/translation products assayed in 100 μl of 200 mM Tris (pH 7.5), 5 mM ATP, 20 mM MgCl2, 2 mM CaCl2, 10 mM NaH2CO3, and 0.5 mM IBMX in the presence of vehicle control or KH7 or YC-1 as specified in the figure legends. Cyclase reactions were incubated at 30°C for 20 min and stopped with 100 μl of 0.2 M HCl. cAMP produced was measured by using the Correlate-EIA Direct cAMP Enzyme Immunoassay Kit (Assay Designs).
Guanylyl Cyclase Assays and cGMP Determinations.
A total of 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. The assay was performed the same way as the AC assay described above. Intracellular cGMP content was determined using Correlate-EIA cGMP Direct Assay (Enzo Life Sciences).
PDE Assays and cAMP Determination.
INS-1E cells were grown in 10-cm dishes to 80% confluence. Cells were lysed in cold lysis buffer [150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 50 mM Tris (pH 8.0)] in the presence of 10 μg/ml aprotinin, 10 μg/ml leupeptin, 5 mM benzamidine, and 1 mM phenylmethanesulfonyl fluoride. To measure PDE activity, 1/20 of total lysate was used per reaction in the presence of 25 pmol of cAMP. Lysates were incubated in 200 mM Tris (pH 7.5), 20 mM MgCl2, and 3 mM dithiothreitol in the presence or absence of IBMX, YC-1, and other PDE inhibitors as indicated in the figure legends for 30 min at 37°C. To stop the assay, 0.2 M HCl was added, and intracellular cAMP content was determined using Correlate-EIA Direct Assay (Enzo Life Sciences). Assays were performed in triplicate.
Western Blot.
Equal numbers of cells were plated on six-well dishes. cAMP accumulation assays were performed in the presence of inhibitors or activators as specified in the figure legends. After 15 min of incubation, 100 μl of Laemmli sample buffer was added directly to the cells in the wells. Ten to fifteen microliters of the sample was resolved by SDS polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with specific antibodies (i.e., anti-phospho-ERK or anti-ERK antibodies).
Results
YC-1 Increases cAMP Production in INS-1E Cells Independent of sGC.
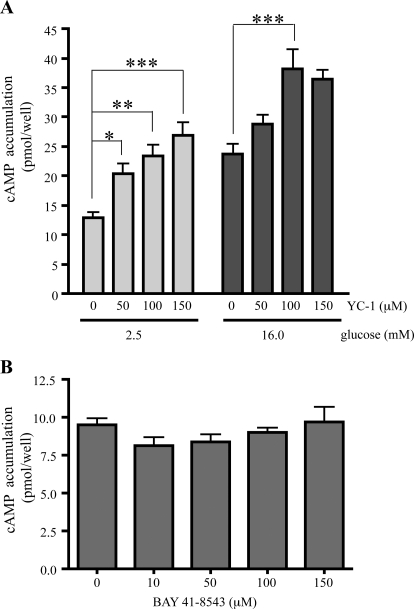
In INS-1E cells, incubation in high (16 mM) glucose elevates intracellular cAMP levels (Ramos et al., 2008). YC-1 increased cAMP accumulation in INS-1E cells in both low (2.5 mM) and high (16 mM) glucose (Fig. 1A). YC-1 stimulation was dose dependent and seemed to be additive with the effect of high glucose. At a concentration of 100 μM, which is the level of YC-1 required for maximal activation of purified sGC (Friebe and Koesling, 1998), cAMP accumulation was stimulated approximately 2-fold compared with the cAMP level in the absence of the drug under both low and high glucose conditions. YC-1-induced cAMP generation was specific to this drug; a structurally unrelated activator of sGC, BAY 41-8543 (Stasch et al., 2002), had no effect on cAMP production in INS-1E cells (Fig. 1B).
Fig. 1.
YC-1, but not BAY 41-8543, potentiates cAMP production in both low and high glucose conditions in INS-1E cells. Two days before the assay, 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. A, total cellular cAMP was measured in INS-1E cells in Krebs-Ringer solution after incubation for 15 min with 2.5 mM glucose or 16 mM glucose in the presence of 0.5 mM IBMX with either vehicle control (0) or with YC-1 at corresponding concentrations. Values represent means ± S.E.M. (n = 4) of total cAMP content per well. B, cAMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose in the presence of 0.5 mM IBMX with either vehicle control or BAY 41-8543. Values represent means ± S.E.M. (n = 3). Analysis of variance statistical analyses were performed with the Bonferroni posttest. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
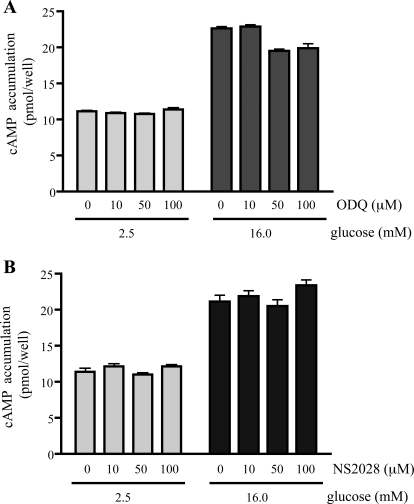
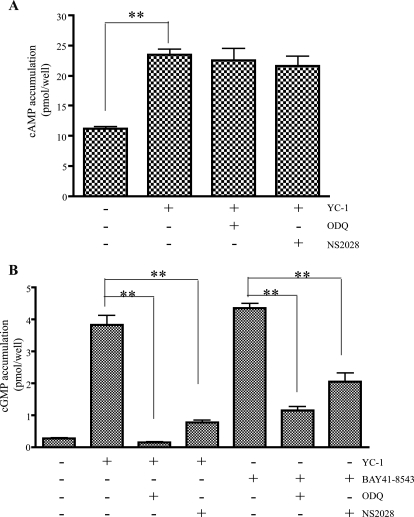
The absence of a cAMP increase stimulated by BAY 41-8543 suggested that the YC-1-induced increase in cAMP production would be independent of sGC. We tested this directly by including two pharmacological inhibitors of sGC, ODQ or NS2028 (Garthwaite et al., 1995; Olesen et al., 1998). After confirming that neither sGC inhibitor affected cAMP levels in INS-1E cells under low or high glucose conditions (Fig. 2), we found that they also had no effect on the YC-1-induced cAMP increase (Fig. 3A). As expected, both YC-1 and BAY 41-8543 stimulated cGMP accumulation in INS-1E cells, and both ODQ and NS 2028 inhibited cGMP production induced by either (Fig. 3B). Thus, YC-1's effect on cAMP appears to be independent of its stimulation of sGC.
Fig. 2.
sGC inhibitors have no effect on glucose-induced cAMP production in INS-1E cells. Two days before the assay, 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. Total cellular cAMP was measured in INS-1E cells in Krebs-Ringer solution with 2.5 mM glucose or 16 mM glucose in the presence of 0.5 mM IBMX or with 2.5 mM glucose or 16 mM glucose with the corresponding amounts of sGC inhibitors ODQ (A) and NS2028 (B). Values represent means ± S.E.M. (n = 3) of total cAMP content per well.
Fig. 3.
sGC inhibitors do not inhibit YC-1-induced cAMP production in INS-1E cells. Two days before the assay, 2.5 × 105 INS-1E cells were plated in each well of a 24-well plate. A, cAMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose in the presence of 0.5 mM IBMX with either vehicle control or 100 μM YC-1 combined with sGC inhibitors, 100 μM ODQ and 100 μM NS2028. B, cGMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose in the presence of 1.0 mM IBMX and 100 μM SNAP with either vehicle control or 100 μM YC-1 or 100 μM BAY 41-8543, combined with sGC inhibitors, 100 μM ODQ and 100 μM NS2028. Values represent means ± S.E.M. (n = 4). Analysis of variance statistical analyses were performed with the Bonferroni posttest. **, P < 0.001.
YC-1 Induces cAMP Accumulation Independent of Its Ability To Inhibit PDE Activity.
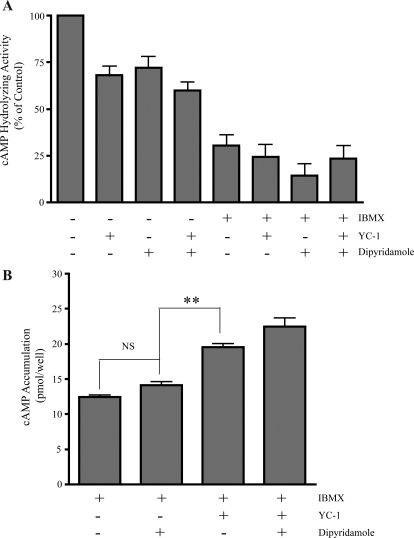
cAMP accumulates as a consequence of an imbalance between second messenger generation by ACs and its degradation by catabolizing PDEs. YC-1 has reported effects on PDE activity (Ko et al., 1994; Galle et al., 1999; Hwang et al., 2008), so we explored whether its ability to elevate intracellular cAMP levels was mediated by the inhibition of a cAMP-catabolizing PDE. To test directly whether YC-1 affected PDE activity in INS-1E cells, we measured cAMP PDE activity in INS-1E lysates in the presence of YC-1 alone or in concert with the broad specificity PDE inhibitor IBMX and the more selective dipyridamole, which potently inhibits the only known IBMX-insensitive cAMP-catabolizing PDE, PDE8 (Fisher et al., 1998; Soderling et al., 1998). YC-1 alone slightly decreased the cAMP PDE activity in INS-1E lysates; its effect was approximately equal to the effect of the PDE8 inhibitor dipyridamole (Fig. 4A). However, the effects of YC-1 were abrogated completely in the presence of 0.5 mM IBMX, suggesting that its effect on PDE activity in INS-1E cells is mediated through the inhibition of an IBMX-sensitive PDE isoform. The cellular cAMP accumulation assays described above (Figs. 1–3) were performed in the presence of IBMX, suggesting that YC-1's effects were not due to PDE inhibition. We retested this by repeating the cellular cAMP accumulation experiments in the presence of both dipyridamole and IBMX. Dipyridamole (in the presence of IBMX) had a slight, but not statistically significant, effect on the level of cAMP accumulation in INS-1E cells, and YC-1 still induced a cAMP increase when both IBMX and dipyridamole were present (Fig. 4B). Therefore, although YC-1 may exhibit some PDE-inhibiting ability, its ability to diminish cAMP-catabolizing activity cannot be solely responsible for its elevation of cAMP levels in whole cells.
Fig. 4.
YC-1-induced cAMP accumulation is not due to PDE inhibition. A, sensitivity of cAMP-hydrolyzing activity in INS-1E lysate to PDE inhibitors, 0.5 mM IBMX and 30 μM dipyridamole, and to 100 μM YC-1. Values represent means ± S.E.M. (n = 4). B, two days before the assay, 2.5.0 × 105 INS-1E cells were plated in each well of a 24-well plate. cAMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose in the presence of 0.5 mM IBMX with either vehicle control or 30 μM dipyridamole and/or with 100 μM YC-1. Values represent means ± S.E.M. (n = 4). Analysis of variance statistical analyses were performed with the Newman-Keuls posttest. **, P < 0.001; NS, P > 0.05.
YC-1-Induced cAMP Production Requires sAC Activity.
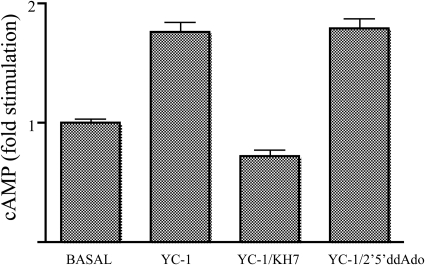
Because YC-1 did not appear to be increasing cAMP levels solely by inhibiting a PDE activity, we hypothesized that it should be stimulating AC activity. In mammalian cells in general and in INS-1E cells in particular (Ramos et al., 2008), there are two classes of ACs: a family of G protein-regulated transmembrane ACs (tmACs) and bicarbonate-, calcium-, and ATP-regulated sAC. We previously established the use of small-molecule inhibitors selective for each class to distinguish which AC was responsible for a specific cAMP signaling cascade (Stessin et al., 2006; Wu et al., 2006; Ramos et al., 2008). KH7 is a small molecule that specifically inhibits sAC (Hess et al., 2005), and the P site ligand 2′,5′-ddAdo is selective for tmACs when used at a concentration ≤50 μM (Johnson et al., 1997; Gille et al., 2004). Using these inhibitors, we demonstrated that in INS-1E cells incretin-stimulated cAMP is generated by tmACs, whereas sAC is responsible for the cAMP induced by elevated glucose (Ramos et al., 2008). Similar to what we observed previously (Ramos et al., 2008), 2′,5′-ddAdo, but not KH7, diminished the “basal” cAMP accumulation in low glucose (cAMP amounts in low glucose in the absence of any inhibitor = 12.976 ± 0.442 pmol/well, in the presence of KH7 = 13.286 ± 0.815 pmol/well, and in the presence of 2′,5′-ddAdo = 9.755 ± 1.384 pmol/well). In contrast, YC-1-induced cAMP was inhibited by KH7 and unaffected by 2′,5′-ddAdo (Fig. 5). Thus, the cAMP increase observed in the presence of YC-1 is dependent upon sAC and not tmACs.
Fig. 5.
YC-1-induced cAMP production is inhibited by a sAC-specific inhibitor. cAMP was measured in INS-1E cells after incubation for 15 min in 2.5 mM glucose in the presence of 0.5 mM IBMX with either vehicle control (basal), 100 μM YC-1, or YC-1 combined either with 30 μM KH7 or 50 μM 2′,5′-ddAdo. The data are presented as fold stimulation of basal cAMP accumulation. Values represent means ± S.E.M. (n = 4).
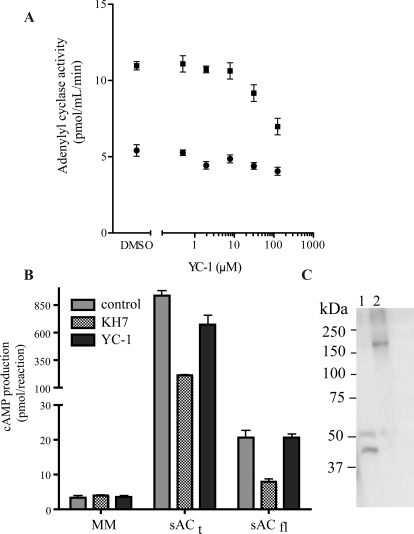
We tested the effect of YC-1 on the activities of the two sAC isoforms, sACt and sACfl, which have been characterized in vitro (Buck et al., 1999; Jaiswal and Conti, 2003; Chaloupka et al., 2006). The truncated and highly active sACt isoform can be expressed heterologously and purified (Chen et al., 2000; Litvin et al., 2003), whereas sACfl has thus far only ever been assayed in whole-cell lysates from transfected cells (Chen et al., 2000; Jaiswal and Conti, 2003; Chaloupka et al., 2006) or in immunoprecipitates (Jaiswal and Conti, 2003). We now demonstrate that active sACfl can be produced and assayed by in vitro transcription/translation systems. In our hands, YC-1 had no effect on basal sACt or sACfl activities, nor did it potentiate calcium and bicarbonate stimulation of sACt (Fig. 6).
Fig. 6.
Activities of the sAC isoforms sACt and sACfl are unaffected by YC-1. A, AC assays were performed using recombinant rat sACt at the indicated concentrations of YC-1 in the presence of 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2 and 10 mM ATP (basal) (circles) or 10 mM MgCl2, 10 mM ATP, 10 mM NaHCO3, and 0.5 mM CaCl2 (suboptimal) (squares) for 30 min at room temperature. Values represent averages of triplicate determinations. B, synthesized sACfl and sACt from in vitro transcription/translation were assayed for AC activity. Cyclase assays were performed in 100 μl of total reaction volume using 15 μl of in vitro transcription/translation products combined with reaction mix composed of 200 mM Tris (pH 7.5), 5 mM ATP, 20 mM MgCl2, 2 mM CaCl2, 10 mM NaH2CO3, and 0.5 mM IBMX with vehicle control or 30 μM KH7 or 100 μM YC-1. MM is the AC reaction conditions alone (no in vitro transcription/translation products). Values represent means ± S.E.M. (n = 3). C, Western blot using the sAC monoclonal antibody R21 to verify the synthesis of sACt (lane 1, 50 kDa) and sACfl (lane 2, 187 kDa) protein in an in vitro transcription/translation system.
YC-1-Induced cAMP Stimulates the MAPK Cascade.
In beta cells, elevated glucose activates the MAPK cascade, and in our previous work, we demonstrated that glucose-induced, sAC-generated cAMP is required for the phosphorylation of the MAPK ERK (Ramos et al., 2008). We now show that the YC-1-induced, sAC-dependent cAMP increase also stimulates the MAPK cascade. YC-1 treatment increases ERK phosphorylation, although not to the same extent as high glucose (Fig. 7). And this YC-1-induced increase is blocked by the sAC inhibitor KH7 but not by the sGC inhibitors ODQ or NS2028. Thus, YC-1 stimulates cAMP accumulation in a manner consistent with elevated glucose; it is dependent upon sAC and results in MAPK cascade activation.
Fig. 7.
Glucose and YC-1 lead to ERK (p42/44) activation in a sAC-dependent manner. Antibodies against phospho-ERK (pERK) or total ERK (ERK) were used for Western blots of INS-1E cells incubated in Krebs-Ringer solution with 16 mM glucose (HG) or 2.5 mM glucose (LG) for 30 min in the presence or absence of 100 μM YC-1 or a combination of YC-1 with 30 μM KH7, 100 μM ODQ, and 100 μM NS2028. Shown are representative experiments repeated multiple times (n = 3).
Discussion
In this study, we demonstrate that YC-1 has effects in INS-1E cells that are independent of its known functions as a sGC activator. We have yet to determine its specific target, but YC-1 induces cAMP accumulation independent of its effects on cGMP generation. The source of the cAMP induced by YC-1 is sAC, and the YC-1 induction of the second messenger occurs when all of the known PDEs are inhibited. These observations reveal that, at least in INS-1E cells, YC-1 increases intracellular cAMP levels via a unique, unknown direct target.
Consistent with its demonstrated ability to stimulate sGC activity, YC-1 activates cGMP accumulation in INS-1E cells. However, using YC-1 to probe intracellular signaling must include confirmation by sGC inhibition (i.e., ODQ or NS 2028) and by independent (and more selective) sGC activation (i.e., by BAY 41-8543). In contrast, YC-1 remains a useful reagent for studying sGC activation in vitro. A great deal of effort has been invested in understanding how it binds to the enzyme, yet where YC-1 binds to sGC remains a matter of debate (Friebe et al., 1999; Derbyshire et al., 2009). Some mutational studies indicated that YC-1 interacts with the catalytic domain of sGC (Russwurm et al., 2002); however, photoaffinity labeling and other mutational studies suggested that it binds within the α1 subunit linker region between the heme-NO and PAS (Per-Arnt-Sim) domains (Stasch et al., 2001; Koglin and Behrends, 2003). Such a mechanism may be consistent with resonance Raman studies, which revealed that YC-1 binding caused a conformational change, which resulted in heme adjustment (Ibrahim et al., 2010).
Galle et al. (1999) showed that YC-1 inhibited the activities of the cGMP-specific PDE5 as well as the activities of the nonselective (i.e., cAMP- and cGMP-catabolizing) PDE1, PDE2, and PDE3 (Galle et al., 1999). Each of these cAMP-catabolizing isoforms is sensitive to inhibition by IBMX (Soderling and Beavo, 2000; Boswell-Smith et al., 2006). There are also cAMP-catabolizing PDEs that are IBMX insensitive (or whose sensitivity to IBMX is unknown). PDE8 is an IBMX-insensitive, cAMP-selective PDE (Soderling et al., 1998), whereas PDE10 and PDE11 are nonselective PDEs whose IBMX sensitivity remains unclear. Fortunately, all three isoforms (along with cGMP-specific PDE5 and PDE6) can be inhibited by dipyridamole (Hetman et al., 2000; Ghosh et al., 2009), and in INS-1E extracts, dipyridamole's small but significant ability to inhibit PDE activity seemed to be additive with that of IBMX. Hwang et al. (2003) showed that YC-1 increased cAMP accumulation in human neutrophils, and because the effect was not seen in the presence of IBMX, they concluded that YC-1 elevated cAMP levels via the inhibition of an IBMX-sensitive PDE. We similarly observed a small, but significant, decrease in cAMP PDE activity in extracts from INS-1E cells in the presence of YC-1. Consistent with YC-1 inhibiting an IBMX-sensitive PDE, the effect of YC-1 seemed to be additive with that of dipyridamole and absent in the presence of IBMX. In any event, in the presence of both IBMX and dipyridamole, YC-1 had no effect on PDE activity in vitro, yet it was still able to stimulate intracellular cAMP accumulation in INS-1E cells. These results suggest that YC-1 affects cAMP accumulation independent of its ability to inhibit PDEs. However, it remains possible that YC-1's inhibitory effects on PDEs are greater or more efficient in vivo than in vitro or that it affects a PDE activity not reflected in the in vitro PDE assay.
The YC-1-induced cAMP elevation is inhibited by the sAC-specific inhibitor KH7, whereas selective inhibition of tmAC activity had no effect. Therefore, YC-1 induction of cAMP requires sAC activity to generate the second messenger. Yet, we were unable to demonstrate that sAC was the target of YC-1; the two biochemically characterized sAC isoforms were inert when tested in vitro. Other sAC isoforms derived from an internal promoter are predicted to exist (Geng et al., 2005; Farrell et al., 2008). These are likely to be distinctly regulated, and they may be the target of YC-1.
It also remains possible that YC-1 increases sAC activity by modulating the intracellular concentration of one of its regulators. sAC catalytic activity is regulated directly by bicarbonate (Chen et al., 2000) and calcium (Jaiswal and Conti, 2003; Litvin et al., 2003), and its affinity for substrate ATP (approximately 1 mM) suggests that it will be sensitive to intracellular fluctuations of ATP (Litvin et al., 2003). All three modulators may contribute to the glucose-induced stimulation of sAC activity in INS-1E cells (Ramos et al., 2008). Glucose metabolism leads to increased intracellular ATP and CO2/bicarbonate levels, and because INS-1E are beta cell-like, the glucose-dependent increase in intracellular ATP level closes the ATP-regulated potassium channel, which depolarizes the cell, opening a voltage-dependent calcium channel and elevating the intracellular calcium level (Ashcroft and Rorsman, 1989; Rutter et al., 1993). We previously demonstrated that the glucose-dependent stimulation of sAC in INS1-E cells is dependent upon the voltage-dependent calcium channel-mediated calcium increase and that the sAC response could be mimicked by potassium depolarization (Ramos et al., 2008). YC-1 can inhibit other types of potassium channels (Park et al., 2010), suggesting that YC-1 may activate sAC subsequent to depolarization-induced calcium entry. However, YC-1 was able to induce approximately the same fold stimulation of cAMP accumulation in both low and high glucose (Fig. 1), implying that glucose and YC-1 increase cAMP via distinct mechanisms. Finally, there are additional modes of regulation of sACfl yet to be understood (Chaloupka et al., 2006), and therefore, it remains possible that YC-1 elevates the intracellular cAMP level via modulation of one of these known, or unknown, sAC activators.
Future studies examining whether YC-1 also elevates cAMP levels in other contexts and whether any observed cAMP increase is sAC dependent and sGC and PDE independent may help to shed light on its precise mechanism of action. In conclusion, we identified an effect of YC-1 that is entirely independent of its sGC-activating properties. Due to such unwanted consequences, caution should be taken in the interpretation of results when using this compound, especially because there are known pathways where cAMP and cGMP modulate the same physiological effect, either oppositely or in concert.
Acknowledgments
We thank Dr. Joe Beavo for advice on the inhibition of PDEs and the members of Buck/Levin laboratory for helpful discussions during the course of this work.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM62328]; the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD059913]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS055255] (all to J.B. and L.R.L.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184135.
- YC-1
- 5-[1-(phenylmethyl)-1H-indazol-3-yl]-2-furanmethanol
- sGC
- soluble guanylyl cyclase
- NO
- nitric oxide
- ERK
- extracellular signal-regulated kinase
- PDE
- phosphodiesterase
- MAPK
- mitogen-activated protein kinase
- sAC
- soluble adenylyl cyclase
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- NS2028
- 8-bromo-4H-2,5-dioxa-3,9b-diaza-cyclopenta[a]naphthalen-1-one
- BAY 41-8543
- 2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-(4-morpholinyl)-4,6-pyrimidinediamine
- SNAP
- S-nitroso-N-acetylpenicillamine
- 2′,5′-ddAdo
- 2′,5′-dideoxyadenosine
- IBMX
- 3-isobutyl-1-methylxanthine
- KH7
- 2-(1H-benzimidazol-2-ylthio)-2-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide propanoic acid
- AC
- adenylyl cyclase
- sACt
- truncated soluble adenylyl cyclase
- sACfl
- full-length soluble adenylyl cyclase
- tmAC
- transmembrane adenylyl cyclase.
Authorship Contributions
Participated in research design: Ramos-Espiritu, Buck, and Levin.
Conducted experiments: Ramos-Espiritu and Hess.
Contributed new reagents or analytic tools: Ramos-Espiritu, Hess, Buck, and Levin.
Performed data analysis: Ramos-Espiritu, Hess, Buck, and Levin.
Wrote or contributed to the writing of the manuscript: Ramos-Espiritu, Buck, and Levin.
References
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178 [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143 [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. (2006) Phosphodiesterase inhibitors. Br J Pharmacol 147:S252–S257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. (1999) Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA 96:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. (2006) Autoinhibitory regulation of soluble adenylyl cyclase. Mol Reprod Dev 73:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles MA, Fanska R, Schmid FG, Forsham PH, Grodsky GM. (1973) Adenosine 3′,5′-monophosphate in pancreatic islets: glucose-induced insulin release. Science 179:569–571 [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289:625–628 [DOI] [PubMed] [Google Scholar]
- Chiang WC, Teng CM, Lin SL, Chen YM, Tsai TJ, Hsieh BS. (2005) YC-1-inhibited proliferation of rat mesangial cells through suppression of cyclin D1-independent of cGMP pathway and partially reversed by p38 MAPK inhibitor. Eur J Pharmacol 517:1–10 [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Fu WM. (2008) Enhancement of active shuttle avoidance response by the NO-cGMP-PKG activator YC-1. Eur J Pharmacol 590:233–240 [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. (2003) Enhancement of long-term potentiation by a potent nitric oxide-guanylyl cyclase activator, 3-(5-hydroxymethyl-2-furyl)-1-benzyl-indazole. Mol Pharmacol 63:1322–1328 [DOI] [PubMed] [Google Scholar]
- Derbyshire ER, Fernhoff NB, Deng S, Marletta MA. (2009) Nucleotide regulation of soluble guanylate cyclase substrate specificity. Biochemistry 48:7519–7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, Buck J. (2008) Somatic ‘soluble’ adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex ‘knockout’ mice. PLoS One 3:e3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DA, Smith JF, Pillar JS, St. Denis SH, Cheng JB. (1998) Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem Biophys Res Commun 246:570–577 [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. (1998) Mechanism of YC-1-induced activation of soluble guanylyl cyclase. Mol Pharmacol 53:123–127 [DOI] [PubMed] [Google Scholar]
- Friebe A, Russwurm M, Mergia E, Koesling D. (1999) A point-mutated guanylyl cyclase with features of the YC-1-stimulated enzyme: implications for the YC-1 binding site? Biochemistry 38:15253–15257 [DOI] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. (1996) Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J 15:6863–6868 [PMC free article] [PubMed] [Google Scholar]
- Galle J, Zabel U, Hübner U, Hatzelmann A, Wagner B, Wanner C, Schmidt HH. (1999) Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol 127:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G, Goodwin DA, Neale S, Riddall D, Garthwaite J. (2002) Soluble guanylyl cyclase activator YC-1 protects white matter axons from nitric oxide toxicity and metabolic stress, probably through Na(+) channel inhibition. Mol Pharmacol 61:97–104 [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. (1995) Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48:184–188 [PubMed] [Google Scholar]
- Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. (2005) Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol 288:C1305–C1316 [DOI] [PubMed] [Google Scholar]
- Ghosh R, Sawant O, Ganpathy P, Pitre S, Kadam VJ. (2009) Phosphodiesterase inhibitors: their role and implications. Int J PharmTech Res 1:1148–1160 [Google Scholar]
- Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, Seifert R. (2004) Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J Biol Chem 279:19955–19969 [DOI] [PubMed] [Google Scholar]
- Grill V, Cerasi E. (1973) Activation by glucose of adenyl cyclase in pancreatic islets of the rat. FEBS Lett 33:311–314 [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman JM, Robas N, Baxendale R, Fidock M, Phillips SC, Soderling SH, Beavo JA. (2000) Cloning and characterization of two splice variants of human phosphodiesterase 11A. Proc Natl Acad Sci USA 97:12891–12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TL, Hung HW, Kao SH, Teng CM, Wu CC, Cheng SJ. (2003) Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol Pharmacol 64:1419–1427 [DOI] [PubMed] [Google Scholar]
- Hwang TL, Zhuo SK, Pan YL. (2008) YC-1 attenuates homotypic human neutrophil aggregation through inhibition of phosphodiesterase activity. Eur J Pharmacol 579:395–402 [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Derbyshire ER, Marletta MA, Spiro TG. (2010) Probing soluble guanylate cyclase activation by CO and YC-1 using resonance Raman spectroscopy. Biochemistry 49:3815–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. (2003) Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA 100:10676–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Désaubry L, Bianchi G, Shoshani I, Lyons E, Jr, Taussig R, Watson PA, Cali JJ, Krupinski J, Pieroni JP, et al. (1997) Isozyme-dependent sensitivity of adenylyl cyclases to P-site-mediated inhibition by adenine nucleosides and nucleoside 3′-polyphosphates. J Biol Chem 272:8962–8966 [DOI] [PubMed] [Google Scholar]
- Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. (1994) YC-1, a novel activator of platelet guanylate cyclase. Blood 84:4226–4233 [PubMed] [Google Scholar]
- Koglin M, Behrends S. (2003) A functional domain of the alpha1 subunit of soluble guanylyl cyclase is necessary for activation of the enzyme by nitric oxide and YC-1 but is not involved in heme binding. J Biol Chem 278:12590–12597 [DOI] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. (2003) Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem 278:15922–15926 [DOI] [PubMed] [Google Scholar]
- Mülsch A, Bauersachs J, Schäfer A, Stasch JP, Kast R, Busse R. (1997) Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br J Pharmacol 120:681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen SP, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, Busse R, Mülsch A. (1998) Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br J Pharmacol 123:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WS, Ko JH, Ko EA, Son YK, Hong da H, Jung ID, Park YM, Choi TH, Kim N, Han J. (2010) The guanylyl cyclase activator YC-1 directly inhibits the voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. J Pharmacol Sci 112:64–72 [DOI] [PubMed] [Google Scholar]
- Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. (2008) Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol 132:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm M, Mergia E, Mullershausen F, Koesling D. (2002) Inhibition of deactivation of NO-sensitive guanylyl cyclase accounts for the sensitizing effect of YC-1. J Biol Chem 277:24883–24888 [DOI] [PubMed] [Google Scholar]
- Rutter GA. (2001) Nutrient-secretion coupling in the pancreatic islet beta-cell: recent advances. Mol Aspects Med 22:247–284 [DOI] [PubMed] [Google Scholar]
- Rutter GA, Theler JM, Murgia M, Wollheim CB, Pozzan T, Rizzuto R. (1993) Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. J Biol Chem 268:22385–22390 [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. (1998) Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA 95:8991–8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA. (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12:174–179 [DOI] [PubMed] [Google Scholar]
- Stasch JP, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Minuth T, Perzborn E, Schramm M, Straub A. (2002) Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vitro studies. Brit J Pharmacol 135:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, et al. (2001) NO-independent regulatory site on soluble guanylate cyclase. Nature 410:212–215 [DOI] [PubMed] [Google Scholar]
- Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. (2006) Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem 281:17253–17258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng CM, Wu CC, Ko FN, Lee FY, Kuo SC. (1997) YC-1, a nitric oxide-independent activator of soluble guanylate cyclase, inhibits platelet-rich thrombosis in mice. Eur J Pharmacol 320:161–166 [DOI] [PubMed] [Google Scholar]
- Tian G, Sandler S, Gylfe E, Tengholm A. (2011) Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes 60:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart P, Malinski T, Ruetten H, Schindler U, Linz W, Schoenafinger K, Strobel H, Wiemer G. (1999) Release of nitric oxide from endothelial cells stimulated by YC-1, an activator of soluble guanylyl cyclase. Br J Pharmacol 128:1316–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Ko FN, Kuo SC, Lee FY, Teng CM. (1995) YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br J Pharmacol 116:1973–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, Levin LR, Jaffrey SR. (2006) Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci 9:1257–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]