Abstract
INTRODUCTION:
High blood pressure during mental stress in subjects with prehypertension is associated with blunted vasodilation in skeletal muscles, which might be improved by an acute bout of exercise.
OBJECTIVE:
To investigate the hemodynamic responses to mental stress before and after a bout of exercise in subjects with prehypertension.
METHOD:
Eighteen subjects with prehypertension and 16 with normotension underwent a mental stress test before and after a maximal cardiopulmonary exercise test on a treadmill. Blood pressure was measured by auscultation, and forearm blood flow was measured by venous occlusion plethysmography; from these measurements, the vascular conductance was calculated.
RESULTS:
Subjects with prehypertension had a higher mean blood pressure during mental stress (prehypertension 112±2 vs. normotension 101±3 mm Hg, p<0.05), and their vascular conductance did not increase (baseline 0.025±0.004 vs. mental stress 0.022±0.003 a.u., p>0.05). After the exercise bout, the mean blood pressure during mental stress was lower in subjects with prehypertension (before exercise 112±2 vs. after exercise 107±2 mm Hg, p<0.05), and vascular conductance increased (baseline 0.011±0.001 vs. mental stress 0.024±0.004 a.u., p<0.05).
CONCLUSION:
Subjects with prehypertension had elevated blood pressure and a blunted vasodilator response during mental stress, but their blood pressure was attenuated and their vasodilator response was normalized after a single bout of maximal dynamic exercise.
Keywords: Psychological stress, Blood pressure, Blood flow, Physical exercise, Hypertension
INTRODUCTION
Arterial hypertension (HT) affects approximately one billion people worldwide, and as the population ages, this number tends to increase.1 The classification of prehypertension (PHT) was introduced in the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. This classification focuses attention on a segment of the population at elevated risk of developing cardiovascular disease and for whom therapies that prevent or delay the onset of HT would be valuable.1
Mental or psychological stress occurs on a daily basis, and the ability of organisms to respond to it is natural and necessary. The cardiovascular system participates extensively in the response to stressful situations, and some of its adjustments involve increases in systemic blood pressure2,3 and vasodilation in skeletal muscles.4,5 However, it has been shown that subjects with PHT have an augmented blood pressure response to mental stress,6-8 which has been associated with an increased risk for the development of HT.2,3 Santangelo et al.6 suggested that one mechanism that might lead to this enhanced pressor response is blunted muscle vasodilation during mental stress. More recently, Schwartz et al.7 found that blunted vasodilation in skeletal muscles in subjects with PHT was not associated with muscle sympathetic nerve activity. Therefore, it is important to assess the impact of factors that might attenuate or reverse the enhanced pressor response and blunted muscle vasodilation during mental stress in subjects with PHT.
Dynamic exercise involving large muscle groups requires complex integrative cardiovascular responses,9 and some effects of exercise on the cardiovascular system can still be observed hours after the exercise ceases.10-12 It has been shown that an acute bout of dynamic exercise decreases blood pressure reactivity to psychosocial stressors in subjects with borderline HT.8,13 However, the mechanism that mediates this response is not known. Specifically, it is not known whether the blunted muscle vasodilation during mental stress in subjects with PHT is improved after a bout of dynamic exercise. Thus, the purpose of this study was to evaluate blood pressure and vascular responses to mental stress experienced before and after a bout of dynamic exercise in subjects with PHT.
MATERIALS AND METHODS
Sample
Subjects were recruited through advertisements in the university and in local newspapers. The sample was composed of 34 subjects, of whom 18 had PHT and 16 had normotension (NT). Eligibility for the study was verified through a clinical history assessment (performed using a questionnaire consisting of questions on cardiovascular history and risk factors for cardiovascular disease),14 a physical examination, two blood pressure measurements per day performed on two different days, biochemical blood analyses, a resting electrocardiogram, and maximal cardiopulmonary exercise testing. All of the subjects fulfilled the following criteria: age between 18 and 49 years, women had regular menstrual cycles, no chronic diseases, no recent infections, body mass index between 18.5 and 29.9 kg/m2, glycemia lower than 5.6 mmol/l,15 total cholesterol lower than 6.21 mmol/l,16 low-density lipoprotein lower than 4.14 mmol/l,16 triglycerides lower than 2.26 mmol/l,16 non-smoker, not using medications other than oral contraceptives, normal resting and exercise electrocardiogram, and sedentary (not engaged in exercise activities lasting 30 minutes or more, three times per week during the last three months). The study procedures were approved by the Ethics Committee of the Antonio Pedro University Hospital, and written informed consent was obtained from all participants prior to the experimental procedures.
Blood pressure assessment
Blood pressure was measured in the right arm in the supine position after ten minutes of quiet rest. Measurements were performed with a calibrated mercury sphygmomanometer and a cuff size that was appropriate for the arm circumference of each subject. According to The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure,1 NT was defined as systolic blood pressure (SBP) <120 mm Hg and diastolic blood pressure (DBP) <80 mm Hg, and PHT was defined as SBP between 120 and 139 and/or DBP between 80 and 89 mmHg. The classification was based on the average of four SBP and DBP measurements, where two measurements were taken during each of two visits to the laboratory. The mean blood pressure (MBP) was calculated with the equation [SBP + (2·DBP)]/3.
Biochemical blood analyses
Plasma glucose was measured with an enzymatic in-vitro test, and serum insulin was determined using an electrochemiluminescence assay. The homeostasis model assessment was calculated to provide an index of insulin resistance.17 Cholesterol and its subfractions, triglycerides and C-reactive protein were determined by the dry chemistry method.
Anthropometric analysis
Anthropometric assessments included measurements of body weight (adult mechanical balance 110 CH, Welmy, Santa Barbora d' Oeste, SP, Brazil) and height followed by a calculation of the body mass index. The waist circumference was obtained with a tape measure at the point midway between the last costal margin and the iliac crest with the subject standing and breathing normally.18
Experimental protocol
After the initial clinical and laboratory screening, there was an experimental visit that occurred in the morning, one hour after a standardized light breakfast with low-fat foods selected by a dietitian.
Women were evaluated from the first until the 12th day after the onset of menstruation. Subjects did not drink alcohol or caffeinated beverages and did not perform intense physical activity for at least 24 hours before the experimental visit. For the experiment, the subjects were placed in supine position in a quiet air conditioned room (±24°C). After instrumentation, they rested quietly for ten minutes. The experimental protocol consisted of blood pressure and forearm vascular reactivity assessments at baseline and during mental stress, and these measurements were performed before and 60 minutes after a maximal cardiopulmonary exercise test.
Hemodynamic measurements
Forearm blood flow (FBF) was measured by venous occlusion plethysmography. The subjects were placed in the supine position with the right arm supported comfortably above the level of the heart. Two cuffs were used: one (8-cm width) was placed around the right wrist, and the other (10-cm width) was placed around the right upper arm. The last cuff was attached to a rapid cuff inflator (EC6; Hokanson, Bellevue, Washington, USA). A mercury-in-silastic strain gauge was placed at the widest girth of the right forearm. The diameter of the strain gauge was 2 cm less than the widest girth of the forearm. The FBF was calculated using a semi-automatic method that has high intra- and inter-evaluator reproducibility (intraclass correlation coefficients between 0.98 and 0.99).19
To measure the BF, the wrist cuff was inflated to 220 mmHg one minute before the onset of the FBF measurement to isolate the vascular circulation of the hand, and it was kept inflated throughout the blood flow measurement.20 At the beginning of all measurements, the evaluator generated a standard calibration pulse of 1 millivolt. The FBF was measured by rapidly inflating the cuff placed around the right upper arm to 50 mmHg (in less than 0.5 s), maintaining this pressure for 10 s and then rapidly deflating it to 0 mmHg for 10 s, thus completing a 20-s cycle. Six cycles of 20 s were performed to determine the baseline FBF, totaling 120 s of baseline measurement. The blood pressure was obtained once during the two minutes of baseline FBF recording.
The measurement of FBF during the mental stress test was similar to that during the baseline measurement, but it was performed in nine 20-s cycles (10-s inflation to 50 mmHg followed by 10-s deflation to 0 mmHg), totaling 180 s of recording of the mental stress FBF. The mean value of each minute of recording was obtained for three cycles, totaling three FBF measurements. Blood pressure was measured three times (once per minute) during the mental stress test using the auscultatory method. The FBF was divided by the mean blood pressure to calculate the forearm vascular conductance (FVC). In addition, the heart rate (HR) was recorded continuously during the mental stress test (Polar Vantage NV, Electro Oy, Kempele, Finland).
Mental stress test
One of the evaluators explained the traditional color-word conflict task (Stroop Color test) to the subjects.21 A continuous auditory conflict with different names of colors was used to increase the difficulty of the mental stress. Each subject was asked to assess the difficulty of the task according to previously established levels of difficulty: 0 = not stressful; 1 = slightly stressful; 2 = stressful; 3 = very stressful; and 4 = extremely stressful.22
Exercise bout
The exercise bout consisted of a standard cardiopulmonary exercise test performed on a treadmill (Master Super ATL, Inbramed, Porto Alegre, RS, Brazil). The protocol was established based on the predicted maximum aerobic capacity (maximal oxygen uptake predicted, based on age, weight, and sex) of each individual.23 The test consisted of a warm-up for three minutes at 3 km/h and 0% grade, followed by a ramp protocol with a linear increase in speed and grade every minute and recovery for five minutes at 4 km/h and 0% grade. The ramp protocol was individualized according to the predicted maximal exercise capacity to reach volitional fatigue at approximately ten minutes. The subjects were verbally encouraged to exercise until exhaustion. All subjects met at least two of the following criteria to confirm that maximal effort was attained: 1) respiratory exchange ratio >1.1; 2) HR within ±10 beats/minute of the age-predicted maximum [210 – (age·0.65)]; 3) a perceived effort score of 10 on the Borg 0-10 scale.24 Ventilation, oxygen uptake, and carbon dioxide output were measured with each breath (CPX Ultima Gas Exchange System; Medgraphics, St Paul, Minnesota, USA). Breath-by-breath ventilation and expired gases were averaged over 20 s to identify the peak oxygen consumption (VO2peak), which was considered to be the highest oxygen uptake value during the exercise bout.
Statistical analysis
The Shapiro–Wilk's test was used to verify the variables' distribution. Group characteristics were compared using Student's t-test, the Mann–Whitney's test or the Chi-square test when appropriate. FBF and FVC data were log-transformed to normalize their distribution. A two-way analysis of variance (ANOVA) adjusted for gender followed by the Newman-Keuls' post hoc test was used to compare hemodynamic data before and after exercise between the NT and PHT groups. Statistical significance was established at p<0.05 in two-tailed comparisons. All analyses were performed using the software STATISTICA (version 8; StatSoft Inc., Tulsa, Oklahoma, USA).
RESULTS
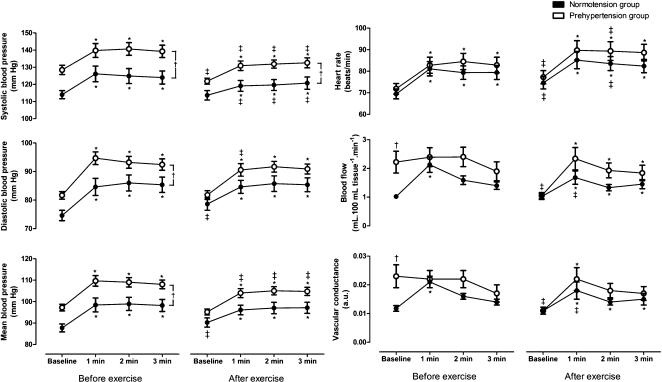
As shown in Table 1, the resting SBP, DBP, and MBP were higher in subjects with PHT than in subjects with NT, as would be expected. However, there were no differences between the groups regarding anthropometric, biochemical, or exercise capacity variables. Figure 1 shows the absolute values of the hemodynamic variables at baseline and during mental stress, which were obtained before and after a single bout of maximal dynamic exercise. Before the exercise bout, the group with PHT had higher FBF and FVC values at baseline than the group with NT. SBP, DBP, MBP, and HR increased during mental stress in both groups, whereas FBF and FVC increased only in the group with NT. In addition, the group with PHT had higher SBP, DBP, and MBP during mental stress compared with the group with NT. After the exercise bout, baseline SBP, FBF, and FVC decreased only in the group with PHT; DBP and MBP increased only in the group with NT; and HR increased in both groups compared with the values obtained before the exercise bout. Furthermore, all variables increased during mental stress in both groups. However, in the PHT group, SBP, DBP, and MBP were lower and HR was higher during the mental stress after the exercise bout than before. In the NT group, SBP was higher but HR, FBF, and FVC were lower during the mental stress after the exercise bout than before. Finally, the SBP during mental stress was higher in the group with PHT than in the group with NT. There was no difference in mental stress scores before and after exercise within the groups (PHT: before exercise 2±0 vs. after exercise 2±2, p = 0.58; NT: before exercise 2±2 vs. after exercise 2±2, p = 0.81).
Table 1.
Anthropometric, biochemical, and hemodynamic characteristics of subjects with prehypertension or normotension.
| Variable | Prehypertension | Normotension | p-value |
| N (male%) | 18 (33.3%) | 16 (12.5%) | 0.15 |
| Age (years) | 34±2 | 33±3 | 0.80 |
| Weight (Kg) | 69.5±2.7 | 68.8±2.4 | 0.44 |
| BMI (Kg/m2) | 25.4±0.5 | 24.9±0.6 | 0.93 |
| Waist circumference (cm) | 80±2 | 81±2 | 0.27 |
| Fasting glucose (mmol/l) | 4.8±0.1 | 4.6±0.1 | 0.55 |
| Fasting insulin (pmol/l) | 41.3±6.8 | 42.8±6.7 | 0.73 |
| HOMA-IR* | 0.9±1.9 | 1.2±0.8 | 0.65 |
| Cholesterol (mmol/l) | 4.6±0.2 | 4.7±0.2 | 0.66 |
| HDL (mmol/l) | 1.5±0.1 | 1.5±0.1 | 0.20 |
| LDL (mmol/l) | 2.6±0.2 | 2.8±0.1 | 0.28 |
| Triglyceride (mmol/l) | 1.1±0.1 | 0.8±0.1 | 0.73 |
| CRP (mg/L)* | 0.4±0.4 | 0.5±0.5 | 0.65 |
| SBP (mm Hg) | 126±2 | 111±2 | <0.01 |
| DBP (mm Hg) | 78±2 | 70±2 | 0.02 |
| MBP (mm Hg) | 95±2 | 84±2 | <0.01 |
| VO2peak (ml/kg min) | 32±2 | 31±2 | 0.67 |
Data are the mean ± SEM or (*) median ± interquartile range. BMI indicates body mass index; HOMA, homeostasis model assessment; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; SBP, systolic blood pressure; DPB, diastolic blood pressure; MBP, mean blood pressure; VO2peak, peak oxygen uptake.
Figure 1.
Absolute hemodynamic responses to mental stress, adjusted for gender, before and after an exercise bout in subjects with prehypertension or normotension. Values are the mean ± SEM. *, p<0.05 vs. within-group baseline; †, p<0.05 vs. same condition between groups; ‡, p<0.05 vs. before-exercise within group.
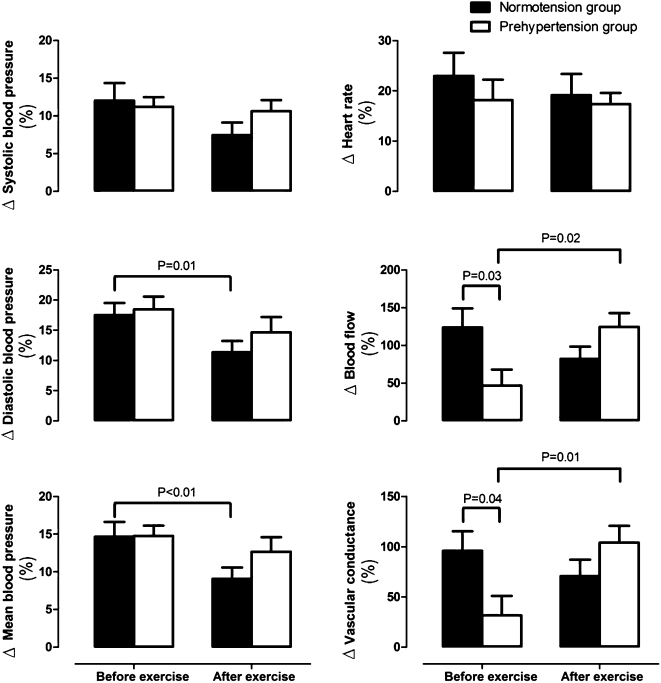
Figure 2 shows the percent change of hemodynamic variables in response to mental stress before and after a single bout of maximal dynamic exercise. Before the exercise bout, FBF and FVC percent change values were lower in the PHT group than in the NT group. After the exercise bout, FBF and FVC percent change values in the PHT group increased relative to the values obtained before the exercise bout, and thus, there was no longer any difference between the groups. It is worth noting that there was 80% power in the FVC percent change analysis, which was the main endpoint of the present study. Moreover, the DBP and MBP percent change values in the NT group decreased compared with the values obtained before the exercise bout, which was not observed in the PHT group.
Figure 2.
Percent change of hemodynamic responses to mental stress, adjusted for gender, before and after an exercise bout in subjects with prehypertension or normotension.
DISCUSSION
The present study investigated hemodynamic responses to a mental stress test performed before and after a bout of maximal dynamic exercise in subjects with PHT or NT. The hypothesis of this study was that subjects with PHT and NT have different levels of vascular reactivity to mental stress and that this difference would disappear after a bout of exercise. This approach revealed two major findings. First, subjects with PHT had higher blood pressure during mental stress than subjects with NT, and the exercise bout reduced their blood pressure during the mental stress test. Second, subjects with PHT presented a blunted vasodilator response to mental stress, and the exercise bout normalized this response.
Previous studies have also shown elevated blood pressure levels during mental stress in subjects with PHT.6-8 However, the mechanisms that mediate this phenomenon are still unclear. Schwartz et al.7 and the present study found that heart rate increases during mental stress were similar in subjects with PHT versus those with NT. However, no study has yet determined whether the increased stroke volume and cardiac output during mental stress are enhanced in subjects with PHT. Therefore, it is possible that increased cardiac output in response to mental stress might contribute to the augmented blood pressure levels in subjects with PHT.
Santangelo et al.6 and Schwartz et al.7 found that part of the enhanced pressor response to mental stress in subjects with PHT was associated with blunted forearm vasodilation. In addition, Scharwrtz et al.7 showed that blunted vasodilation in response to mental stress in subjects with PHT was not mediated by sympathetic nerve activity in the muscle. In the present study we also observed an attenuated forearm vasodilator response to mental stress, which corroborates previous findings.
Subjects with PHT had a higher blood flow and vascular conductance at rest, which reduced their vasodilator reserve (i.e., the change from baseline to maximal vasodilation), thus impairing their vasodilator response to mental stress. A reduction in the vasodilator reserve was shown previously in the forearm7 and coronary25 circulation of subjects with PHT, and this reduction seems to be partially mediated by passive arterial distention as a consequence of increased perfusion pressure.26 Additionally, this mechanism seems to present at the onset of HT pathophysiology.27 Further evolution of the disease induces arterial remodeling to decrease the tensile stress in the arterial wall,27 which occurs by means of an increase in the intima-media thickness and a decrease in the vessel lumen.26
The increased blood flow in the muscle vascular bed during mental stress is dependent on the integrity of the vascular endothelium, and nitric oxide (NO) production by the endothelium seems to be a cornerstone mechanism. Studies have shown that during mental stress, sympathetic cholinergic fibers,4 beta 2-adrenergic receptor stimulation by adrenaline28,29 and increased shear stress30 stimulate the release of NO, leading to vasodilation. Thus, when NG-monomethyl-L-arginine was administered to healthy subjects to inhibit NO production, the increase in forearm vascular conductance during mental stress was blunted by approximately 45%5 to 65%.4 In this context, it was shown that subjects with HT have impaired NO-dependent vasodilation during mental stress. Considering that there is evidence that subjects with PHT also present a certain degree of endothelial dysfunction,31 it is conceivable that impaired endothelial-dependent vasodilation might also be involved in the blunted vasodilation during mental stress in subjects with PHT.
The present study showed that the enhanced pressor response to mental stress in subjects with PHT was attenuated after a bout of exercise, which is consistent with similar findings from a meta-analysis that evaluated 15 randomized controlled studies that investigated the acute effects of aerobic exercise on blood pressure responses to mental stress in subjects with NT and HT.13 Boone et al.8 and the present study found that the HR response to mental stress was not affected by an acute exercise bout in subjects with PHT. No study has yet evaluated stroke volume and cardiac output responses to mental stress in this population following an exercise bout. However, West et al.32 observed a significant reduction in the total vascular resistance during mental stress in subjects with borderline HT after an exercise bout. In this context, the present study adds the finding that the forearm vasodilator response during mental stress was enhanced after an acute bout of exercise in subjects with PHT, which suggests that there was enhanced vasodilation in the forearm skeletal muscles.
Three primary mechanisms might be involved in the enhanced vasodilation in response to mental stress in skeletal muscles following an acute bout of exercise. First, it was observed that the exercise bout reduced SBP at rest in subjects with PHT, which might have attenuated the passive arterial distension caused by increased perfusion pressure27 and thus might be associated with the reduction in baseline blood flow. However, regardless of the cause, reduced baseline blood flow increases the vasodilator reserve, thereby facilitating vasodilation during mental stress. Second, there is evidence that following a bout of dynamic exercise, endothelial-dependent vasodilation is improved, even in vascular bed segments that were not directly involved in the exercise.12,33 This phenomenon has been attributed to a systemic increase in the expression and phosphorylation of endothelial nitric oxide synthase34,35 as a consequence of the systemic increase in shear stress during exercise, which leads to an increase in the bioavailability of NO.33 In addition, it was shown that beta 2- adrenergic receptor responsiveness is enhanced after an acute bout of exercise,36 which can also improve the vasodilator response to mental stress. Third, Brownley et al.36 showed that a reduced noradrenaline response to a stress task was associated with an attenuated post-exercise stress-related blood pressure response. Therefore, it is possible that a reduction in sympathetic vasoconstrictor activity might also have contributed to this effect.
The results of this study have some clinical implications. The novel result of this study is that a single bout of exercise reversed an impaired vasodilator response to mental stress, as indicated by a lower blood pressure increase. Considering that an enhanced blood pressure response to mental stress is associated with a greater risk for the development of cardiovascular disease, our results suggest that by correcting the vasodilator response to mental stress, as the exercise bout did, it is possible to attenuate the blood pressure increase during mental stress in subjects with PHT. Further studies should determine whether chronic exposure to exercise leads to vascular adaptations that correct the vasodilator response, and whether other non-pharmacological vasodilatory interventions such as anti-oxidant and/or L-arginine supplements would yield similar benefits.
The results presented here should be interpreted considering certain limitations. Blood flow was measured by venous occlusion plethysmography, which takes into account the blood flow to all tissues in the evaluated segment. The tissue that contributes most to the total blood flow measured by plethysmography is skeletal muscle,37 but skin blood flow can also contribute to the results to a small extent.37 Therefore, the temperature in the experimental room was maintained at a fairly constant level to minimize variations in skin blood flow. This technique has been validated,38 and is widely used to measure blood flow in skeletal muscle. It is possible that repeated exposure to mental stress could lead to habituation. However, Hammer et al. showed that repeated exposure to the same test that was used in the present study did not change cardiovascular responses to mental stress.39 In addition, in the present study, there was no change in stress perception scores before and after exercise in either group. Hence, a repeated exposure to mental stress might not have influenced the results that were observed in the present study. Another limitation is the low number of men in the sample. This limitation is minimal because there was no significant difference in the number of men between the groups. Finally, we used a maximal dynamic exercise test to investigate the effects of exercise on cardiovascular responses to mental stress. This method has the advantage of providing a standardized exercise stimulus. It was shown previously that a bout of maximal dynamic exercise increases endothelial-mediated vasodilation for at least 60 minutes12 and increases the systemic production of NO,33 which supports our study mechanistically. However, this exercise protocol is not used during training programs. Thus, further studies should investigate whether a regular session of aerobic exercise training yields similar benefits in subjects with PHT.
CONCLUSION
The present study showed that subjects with PHT have elevated blood pressure and blunted vasodilation during mental stress compared with subjects with NT, and a single bout of maximal dynamic exercise reduced their blood pressure and normalized their vasodilator response to mental stress.
ACKNOWLEDGMENTS
The authors thank Felipe de Sá Pereira, Thales Coelho Barbosa, Tatiane Marinz de Souza and Rogério Barbosa M. Barros for their assistance during the experiments and Labs D'OR for biochemical blood analyses.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 2.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–203. doi: 10.1161/01.CIR.0000143840.77061.E9. 10.1161/01.CIR.0000143840.77061.e9",-1,"xxx/77061.e9 [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–8. doi: 10.1161/01.CIR.0000133415.37578.E4. 10.1161/01.CIR.0000133415.37578.e4",-1,"xxx/37578.e4 [DOI] [PubMed] [Google Scholar]
- 4.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–8. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol. 1997;80:1070–4. doi: 10.1016/s0002-9149(97)00605-x. 10.1016/S0002-9149(97)00605-X [DOI] [PubMed] [Google Scholar]
- 6.Santangelo K, Falkner B, Kushner H. Forearm hemodynamics at rest and stress in borderline hypertensive adolescents. Am J Hypertens. 1989;2:52–6. doi: 10.1093/ajh/2.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz CE, Durocher JJ, Carter JR. Neurovascular responses to mental stress in prehypertensive humans. J Appl Physiol. 2011;110:76–82. doi: 10.1152/japplphysiol.00912.2010. 10.1152/japplphysiol.00912.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone JB, Jr, Probst MM, Rogers MW, Berger R. Postexercise hypotension reduces cardiovascular responses to stress. J Hypertens. 1993;11:449–53. doi: 10.1097/00004872-199304000-00017. 10.1097/00004872-199304000-00017 [DOI] [PubMed] [Google Scholar]
- 9.Souza Nery S, Gomides RS, da Silva GV, de Moraes Forjaz CL, Mion D, Jr, Tinucci T. Intra-arterial blood pressure response in hypertensive subjects during low- and high-intensity resistance exercise. Clinics. 2010;65:271–7. doi: 10.1590/S1807-59322010000300006. 10.1590/S1807-59322010000300006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso CG, Jr, Gomides RS, Queiroz AC, Pinto LG, da Silveira Lobo F, Tinucci T, et al. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics. 2010;65:317–25. doi: 10.1590/S1807-59322010000300013. 10.1590/S1807-59322010000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobrega AC. The subacute effects of exercise: concept, characteristics, and clinical implications. Exerc Sport Sci Rev. 2005;33:84–7. doi: 10.1097/00003677-200504000-00005. 10.1097/00003677-200504000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Bousquet-Santos K, Soares PP, Nobrega AC. Subacute effects of a maximal exercise bout on endothelium-mediated vasodilation in healthy subjects. Braz J Med Biol Res. 2005;38:621–7. doi: 10.1590/s0100-879x2005000400017. 10.1590/S0100-879X2005000400017 [DOI] [PubMed] [Google Scholar]
- 13.Hamer M, Taylor A, Steptoe A. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol. 2006;71:183–90. doi: 10.1016/j.biopsycho.2005.04.004. 10.1016/j.biopsycho.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine Position Stand and American Heart Association. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Med Sci Sports Exerc. 1998;30:1009–18. 10.1097/00005768-199806000-00034 [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–8. [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 18.Chan DC, Watts GF, Barrett PH, Burke V. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM. 2003;96:441–7. doi: 10.1093/qjmed/hcg069. 10.1093/qjmed/hcg069 [DOI] [PubMed] [Google Scholar]
- 19.Silva BM, Neves FJ, Rocha NG, Cagy M, de Souza MN, Nobrega AC. Intra- and inter-tester reproducibility of venous occlusion plethysmography: comparison between a manual and a semi-automatic method of blood flow analysis. Physiol Meas. 2009;30:1267–79. doi: 10.1088/0967-3334/30/11/010. 10.1088/0967-3334/30/11/010 [DOI] [PubMed] [Google Scholar]
- 20.Burggraaf J, Kemme MJ, Muller LM, Schoemaker RC, Cohen AF. The influence of the hand circulation on the assessment of venous distensibility of the human forearm with venous occlusion plethysmography. Br J Clin Pharmacol. 2000;50:621–3. doi: 10.1046/j.1365-2125.2000.00307.x. 10.1046/j.1365-2125.2000.00307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroop JP. Studies in interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. 10.1037/h0054651 [Google Scholar]
- 22.Avila WS, Calil OA, Trombetta IC, Negrao CE, Grinberg M, Zugaib M, et al. Vascular reactivity response to mental stress in pregnant women with mitral stenosis. Arq Bras Cardiol. 2006;87:128–36. doi: 10.1590/s0066-782x2006001500010. 10.1590/S0066-782X2006001500010 [DOI] [PubMed] [Google Scholar]
- 23.Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:1451; author reply. doi: 10.1164/ajrccm.167.10.950. [DOI] [PubMed] [Google Scholar]
- 24.Midgley AW, McNaughton LR, Polman R, Marchant D. Criteria for determination of maximal oxygen uptake: a brief critique and recommendations for future research. Sports Med. 2007;37:1019–28. doi: 10.2165/00007256-200737120-00002. 10.2165/00007256-200737120-00002 [DOI] [PubMed] [Google Scholar]
- 25.Laine H, Raitakari OT, Niinikoski H, Pitkanen OP, Iida H, Viikari J, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32:147–53. doi: 10.1016/s0735-1097(98)00222-8. 10.1016/S0735-1097(98)00222-8 [DOI] [PubMed] [Google Scholar]
- 26.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–93. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 27.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003;89:1104–9. doi: 10.1136/heart.89.9.1104. 10.1136/heart.89.9.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von zur Muhlen B, Millgard J, Sarabi M, Lind L. Ambulatory blood pressure and endothelium-dependent vasodilation in hypertensive patients. Blood Press. 2000;9:110–5. doi: 10.1080/080370500453438. 10.1080/080370500453438 [DOI] [PubMed] [Google Scholar]
- 29.Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol. 1997;504:211–20. doi: 10.1111/j.1469-7793.1997.211bf.x. 10.1111/j.1469-7793.1997.211bf.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reims HM, Sevre K, Hoieggen A, Fossum E, Eide I, Kjeldsen SE. Blood viscosity: effects of mental stress and relations to autonomic nervous system function and insulin sensitivity. Blood Press. 2005;14:159–69. doi: 10.1080/08037050510034176. 10.1080/08037050510034176 [DOI] [PubMed] [Google Scholar]
- 31.Cugini P, Baldoni F, De Rosa R, Pandolfi C, Colotto M, Buccarella PA, et al. Higher blood pressure load (baric impact) in normotensives with endothelial dysfunction: a paraphysiological status of “pre-hypertension”. Clin Ter. 2002;153:309–15. [PubMed] [Google Scholar]
- 32.West SG, Brownley KA, Light KC. Postexercise vasodilatation reduces diastolic blood pressure responses to stress. Ann Behav Med. 1998;20:77–83. doi: 10.1007/BF02884452. 10.1007/BF02884452 [DOI] [PubMed] [Google Scholar]
- 33.Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radic Biol Med. 2005;38:1164–9. doi: 10.1016/j.freeradbiomed.2004.12.018. 10.1016/j.freeradbiomed.2004.12.018 [DOI] [PubMed] [Google Scholar]
- 34.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–96. doi: 10.1074/jbc.M108789200. 10.1074/jbc.M108789200 [DOI] [PubMed] [Google Scholar]
- 35.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587:3911–20. doi: 10.1113/jphysiol.2009.172916. 10.1113/jphysiol.2009.172916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brownley KA, Hinderliter AL, West SG, Girdler SS, Sherwood A, Light KC. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med Sci Sports Exerc. 2003;35:978–86. doi: 10.1249/01.MSS.0000069335.12756.1B. 10.1249/01.MSS.0000069335.12756.1B [DOI] [PubMed] [Google Scholar]
- 37.Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol. 1955;128:258–67. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. 10.1136/bmj.300.6719.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamer M, Boutcher YN, Park Y, Boutcher SH. Reproducibility of skeletal muscle vasodilatation responses to Stroop mental challenge over repeated sessions. Biol Psychol. 2006;73:186–9. doi: 10.1016/j.biopsycho.2006.03.003. 10.1016/j.biopsycho.2006.03.003 [DOI] [PubMed] [Google Scholar]