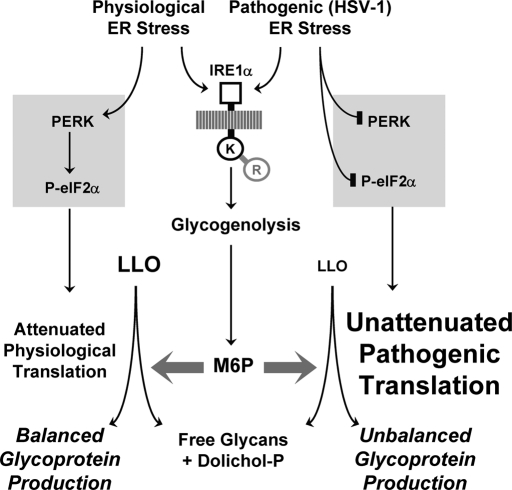
FIGURE 11:
LLO destruction and depletion by M6P signaling during a pathogenic UPR. LLO destruction involves UPR signaling by the IRE1α kinase domain, and mobilization of M6P from glycogen. M6P then promotes LLO cleavage, yielding dolichol-P and lumenal free glycans, possibly by stimulating a hydrolytic activity of OT. PERK activation by conventional ER stress attenuates translation, and reduces LLO consumption for N-glycoprotein synthesis. This offsets the effect of the IRE1α signal for LLO destruction, which by itself is insufficient to suppress LLO levels. Thus, N-glycosylation of endogenous proteins remains balanced. In contrast, inhibition of PERK signaling to disable host defenses during HSV-1-induced stress removes the brake on N-glycoprotein synthesis, resulting in unabated LLO consumption. In combination with LLO destruction, this suppresses LLO levels, and envelope protein N-glycosylation becomes unbalanced. In this way, LLO destruction is not detrimental with conventional/physiological ER stresses, but may penalize invading pathogens for blocking translation-dependent host defenses.