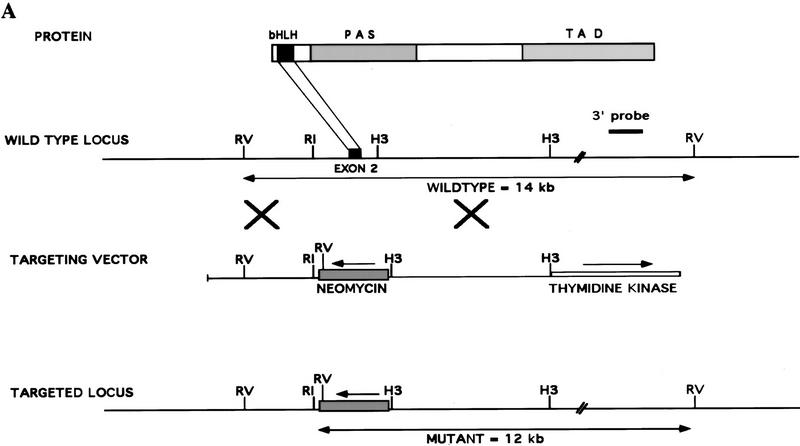
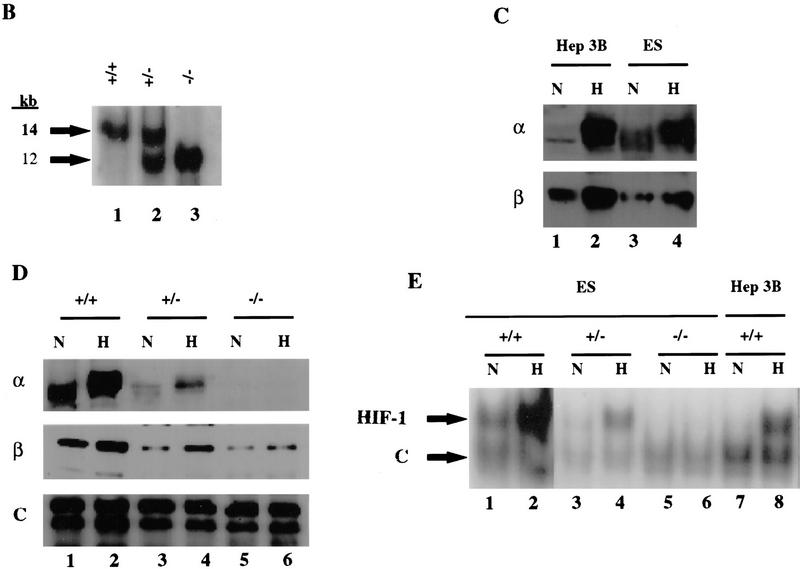
Figure 1.
Targeted disruption of the Hif1a gene by homologous recombination in ES cells. (A) Structure of HIF-1α protein, wild-type Hif1a locus, targeting vector, and targeted Hif1a locus. Important functional domains in the protein are the bHLH and PAS domains, which are required for dimerization and DNA binding, and the transactivation domains (TAD). Homologous recombination (large crosses) results in replacement of exon 2 with a neomycin resistance gene in the opposite transcriptional orientation (arrow). Targeted clones are G418 and gancyclovir resistant owing to the presence of the neomycin-resistance gene and the absence of the thymidine kinase gene. (B) DNA blot hybridization analysis of ES cell clones. The wild-type and targeted loci are identified by the presence of 14- and 12-kb EcoRV restriction fragments, respectively, that are detected by the 3′ probe shown in A. (C) HIF-1 immunoblot analysis of Hep3B and ES cells. Nuclear extracts were prepared from cells cultured under nonhypoxic (N) (20% O2) or hypoxic (H) (1% O2) conditions for 4 hr. Immunoblot assays were performed using affinity-purified antibodies specific for HIF-1α (top) or HIF-1β (bottom). (D) Immunoblot analysis of HIF-1 expression in Hif1a+/+, Hif1a+/-, and Hif1a−/− ES cells. Nuclear extracts were prepared from cells cultured under nonhypoxic (N) or hypoxic (H) conditions for 4 hr. Immunoblot assays were performed using antibodies specific for HIF-1α (top), HIF-1β (middle), or a control (C) protein, topoisomerase I (bottom). (E) Electrophoretic mobility-shift assay of HIF-1 DNA-binding activity. Nuclear extracts from ES and Hep3B cells were incubated with a double-stranded oligonucleotide probe containing an 18-bp EPO gene sequence. Binding of HIF-1 and a constitutively expressed factor (C) is indicated.