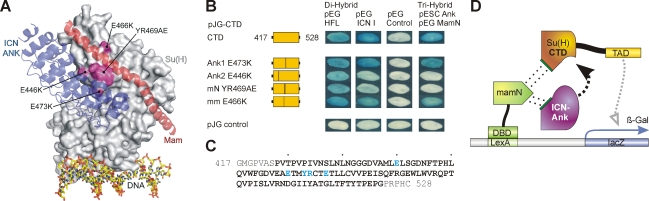
FIGURE 2:
Fine mapping of the Hairless contact site on Su(H) CTD. (A) Structure of the human CSL-ICN-Mam activator complex (PDB ID: 2F8X) (Nam et al., 2006). CSL is represented as a gray surface, and ICN and Mam are colored blue and red, respectively. Mutations in the CTD that lie at the interface with ICN and Mam are colored magenta. (B) Mutant CTD constructs were tested in a yeast two-hybrid assay for binding to full-length Hairless (HFL) and to intracellular Notch (ICN I). Moreover, the mutant CTD constructs were tested in a yeast three-hybrid assay for their potential to assemble the trimeric activator complex with Notch ANK and Mam. Empty vectors served as negative controls. Position of mutations within CTD is indicated. Note that mutations Ank2 and mN are competent for binding HFL but are considerably reduced for ICN binding and also fail to assemble the trimeric activator complex. Hence, Notch and Hairless contact different sites on Su(H). (C) Primary sequence of the Su(H)–CTD construct; the CTD is shown in bold. Amino acids interacting with ANK/Mam that were mutated are colored blue. (D) Schematic for yeast three-hybrid assay; the N-terminal domain of Mam (mamN) was fused to the LexA DNA-binding domain, and the CTD of Su(H) was fused with the trans-activation domain (TAD). Notch ANK was provided from the plasmid pESC. Su(H) CTD constructs carried mutations as indicated in B. Assembly of the trimeric complex results in activation of the lacZ reporter.