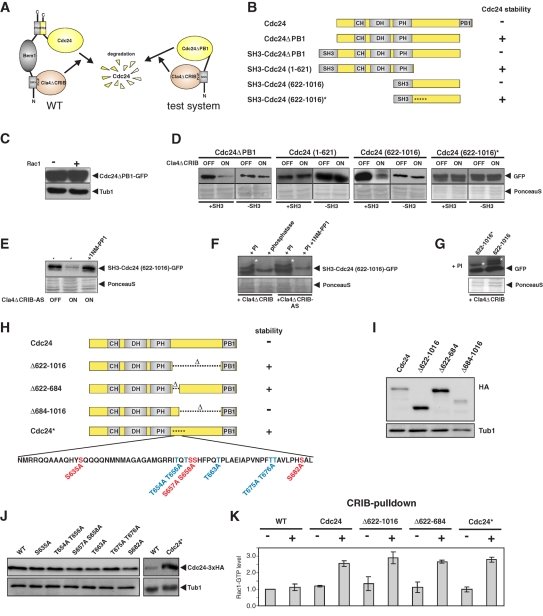
FIGURE 4:
Characterization of the region within Cdc24 that confers Cla4-dependent destabilization. (A) Schematic representation of the test system for Cdc24 fragments that are important for Cla4-induced Cdc24 destabilization. Bem1 normally interacts with Cdc24 via its PB1 domain and with Cla4 via an SH3 domain. The “bridging” function of Bem1 is mimicked by direct fusion of the SH3 domain of Bem1 to the Cdc24 fragments. (B) Schematic overview of the tested Cdc24 fragments. (C) Protein stability of ectopically expressed mutant Cdc24ΔPB1-GFP was determined in the presence (+) and absence (−) of Rac1. (D) Western blot analysis of C-terminal GFP fusions of the indicated Cdc24 fragments. Cdc24 fragments were expressed in Δrac1 mutant cells as N-terminal fusions to the SH3 domain of Bem1 (+SH3). Cdc24 fragments without the SH3 domain (−SH3) served as control. Probes were taken before (OFF) and after 4 h of Cla4ΔCRIB overexpression (ON). (E) Determination of the protein level of fragment SH3-Cdc24(622-1016)-GFP before (OFF) and 6 h after induction of the overexpression of the analogue-sensitive variant of Cla4ΔCRIB (Cla4ΔCRIB-AS), which carries the M629A mutation. Cultures were grown in the presence and absence of the inhibitor 1NM-PP1. (F) Detection of mobility shifts in SH3-Cdc24(622-1016)-GFP caused by expression of Cla4ΔCRIB or Cla4ΔCRIB-AS. Protein extracts of kinase-overproducing cultures were prepared in the presence of phosphatase inhibitors (PIs) or after addition of ALP. Cla4ΔCRIB-AS was expressed in the presence and absence of the inhibitor 1NM-PP1. Asterisks mark phosphorylated SH3-Cdc24(622-1016)-GFP. (G) Wild-type SH3-Cdc24(622-1016)-GFP and the stabilized protein SH3-Cdc24(622-1016)*-GFP were prepared in the presence of PIs shortly after induction of Cla4ΔCRIB overexpression. Mobility shifts are indicated by asterisks. (H) Schematic representation of internal Cdc24 deletions that were introduced at the genomic locus. In Cdc24* all serine (S) and threonine (T) residues of the indicated domain were exchanged for alanine (I). Protein levels of the indicated Cdc24 mutant proteins. For visualization, the proteins were C-terminally fused to a 3xHA tag. (J) Western blot analysis of the protein levels of Cdc24 mutants carrying the indicated mutations. (K) Quantification of active Rac1-GTP before (−) and after (+) 4 h of the overexpression of the indicated Cdc24 variants. The diagram shows the mean values of three independent determinations. SDs are indicated.