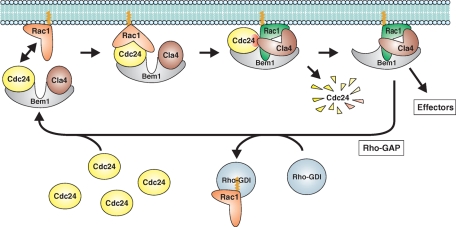
FIGURE 6:
Schematic model of the negative feedback loop that triggers destruction of the Rac1-specifc Rho-GEF Cdc24. Cdc24 and Cla4 are assumed to be associated with the scaffold protein Bem1. Inactive GDP-loaded Rac1 (orange) is recruited into this complex by Cdc24. Within this transient quaternary complex Cdc24 activates Rac1 by stimulating GDP/GTP cycling. In its GTP-bound form, Rac1 (green) interacts with Cla4 and induces a conformational change in Cla4 (open shape). This relieves autoinhibition of the kinase domain and results in phosphorylation of Cdc24 (red dot). Phosphorylated Cdc24 is rapidly destroyed, most probably by proteasomal degradation. This results in the release of an active Bem1–Rac1–Cla4 complex, which triggers polar growth by interaction with yet-unknown effectors. Recycling of Bem1 and Rac1 is accomplished by interaction with Rac1-specific Rho-GAP and Rho-GDI proteins.