Abstract
The Selvester QRS score translates subtle changes in ventricular depolarization measured by the electrocardiogram (ECG) into information about myocardial scar location and size. This estimation of scar has been shown to have a high degree of correlation with autopsy-measured myocardial infarct (MI) size. Additionally, multiple studies have demonstrated the value of the QRS score in post-MI patients to provide prognostic information. Recent studies have demonstrated that increasing QRS score is predictive of increased implantable defibrillator (ICD) shocks for ventricular tachycardia and fibrillation as well as decreased response to cardiac resynchronization therapy (CRT). While QRS scoring has never achieved widespread clinical use, increased interest in patient selection and risk-stratification techniques for ICDs and CRT has led to renewed interest in QRS scoring and its potential to identify which patients will benefit from device therapy. The QRS score criteria were updated in 2009 to expand their utility to a broader population by accounting for the different ventricular depolarization sequences in patients with bundle branch/fascicular blocks or ventricular hypertrophy. However, these changes also introduced additional complexity and nuance to the scoring procedure. This paper provides detailed instructions and examples on how to apply the QRS score criteria in the presence of confounding conduction types to facilitate understanding and enable development and application of automated QRS-scoring.
Introduction
In an era of multimodal cardiac imaging, understanding the information contained in the 12-lead electrocardiogram (ECG) is important yet frequently overlooked. The Selvester QRS score, which was described in preliminary form 1972,1 translates subtle changes in cardiac electrical activity into information about myocardial scar location and size.2,3 While QRS scoring was rigorously validated in comparison to autopsy-measured myocardial infarct (MI) size in the 1980s4–7 and shown to have prognostic value,3,8–14 this ECG method did not achieve widespread clinical use. However, the need to improve patient selection for implantable defibrillators (ICDs) and cardiac resynchronization therapy (CRT) has led to a renewed interest in QRS scoring because characterizing myocardial scar may better predict which patients will benefit from ICDs and CRT.15,16
The older versions of QRS scoring could only be used in the absence of ECG confounders (i.e. bundle branch/fascicular blocks and ventricular hypertrophy), which makes QRS scoring impractical for ICD/CRT patient selection because greater than 50% of potential ICD patients and nearly all CRT patients have ECG confounders.2,15,17 In 2009, new QRS-score criteria for use in the presence of ECG confounders were published and shown to be able to identify and quantify myocardial scar in comparison to contrast-enhanced magnetic resonance imaging in patients with ischemic and non-ischemic cardiomyopathy,2,8 including patients with Chagas’ heart disease.3 Subsequent studies have shown that these QRS-score criteria for use in the presence of ECG confounders can enhance prediction of which patients will experience appropriate ICD shocks and respond to CRT.15,16 The purpose of this report is to provide more detailed instructions beyond the 2009 publication for classifying the type of ECG confounder and applying the QRS-score criteria in order to better facilitate manual and automated application.
Review of Modifications to QRS Scoring
The QRS score has been updated and amended multiple times since its original description in 1972.1 The most recent changes came in 2009 with a publication describing how to apply the QRS score in the setting of hypertrophy and conduction defects.8 Prior to this study, fascicular blocks, bundle branch blocks and hypertrophy were considered confounding factors that prevented infarction evaluation via QRS score. The updated criteria in 2009 account for the different activation sequences and conduction properties associated with these defects and are able to distinguish the additional electrical changes associated with infarction. This was made possible by using adjusted criteria for each of the following abnormalities: left and right ventricular hypertrophy (LVH, RVH), left anterior fascicular block (LAFB), left bundle branch block (LBBB), right bundle branch block (RBBB), RBBB + LAFB, and no confounders. The scoring criteria sets differ slightly among all of these abnormalities with LBBB having the most dissimilar criteria, but they still follow the same principle of a score with each point representing 3% infarct of the left ventricular (LV) myocardium.
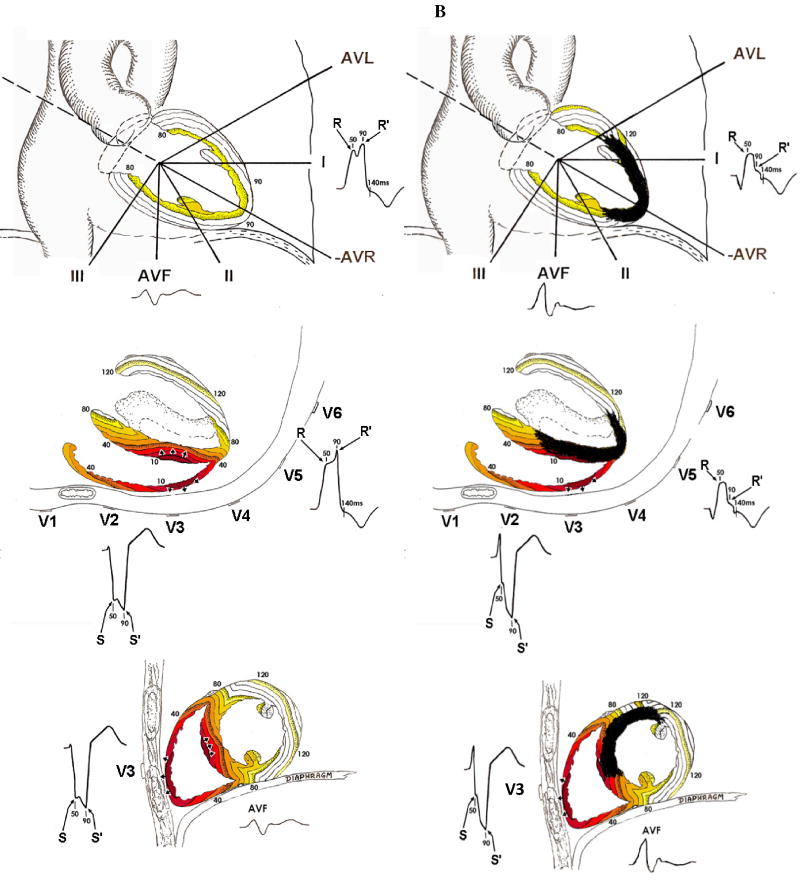
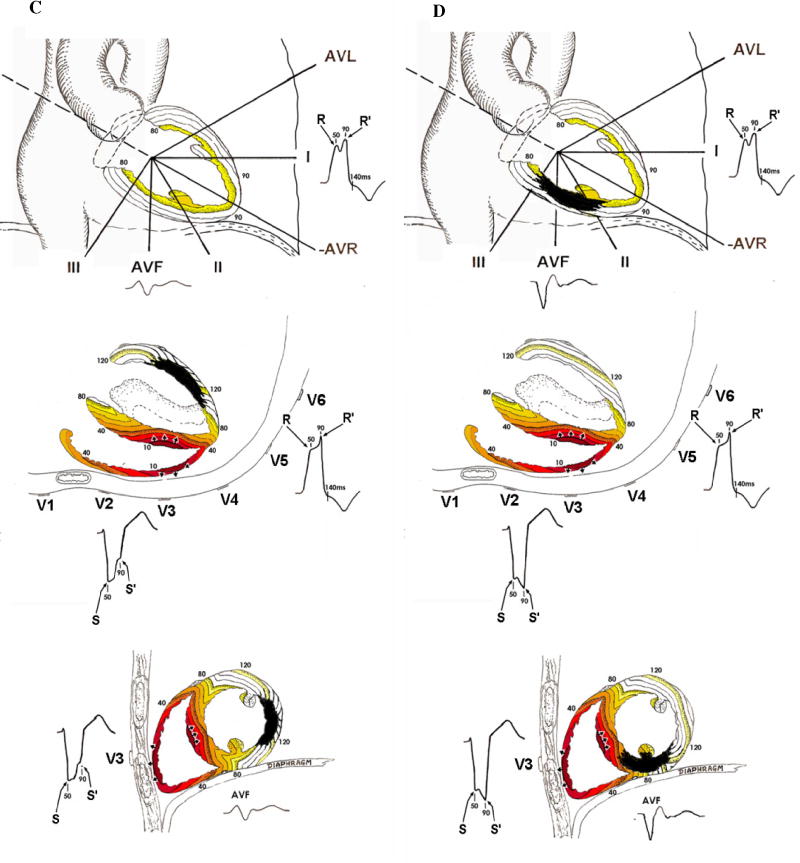
For example, in LBBB the QRS scoring criteria for the limb leads (I, II, aVL and aVF) are similar to those in all other ECGs; however, the precordial lead scoring criteria are dramatically different. In normal conduction, anteroseptal scar causes the rest of the LV to activate largely unopposed producing net posteriorly directed electrical activity and creating a Q wave in leads V1-V3. Conversely in LBBB in the absence of scar, the LV is activated via electrical depolarization from the right ventricle (RV) through the septum and finally out to the posterolateral LV wall creating a small or absent initial R wave and deep Q or S wave in V1 and V2 (Figure 1A). Anteroseptal scar in the presence of LBBB causes depolarization from the RV to the LV through the septum to be impeded and the initial electrical forces are directed anteriorly due to the unopposed activation of the RV free wall resulting in large R waves in V1 and V2 (Figure 1B).8 Thus, anteroseptal scar results in Q wave formation in normal conduction, but causes large R wave formation in LBBB. Posterolateral scar disrupts the late forces of the QRS complex as depolarization spreads from the septum out to the posterolateral wall causing notching of the terminal portion of the S wave in V1 and V2 and, if the scar extends toward the apex, of the R wave in V5 and V6 (Figure 2C). Inferior scar behaves similarly in LBBB as other conduction types causing a Q wave in aVF due to unopposed superior activation early in ventricular activation (Figure 2D).
Figure 1. LBBB activation patterns.
All panels demonstrate the ventricular activation pattern in LBBB and ECG wave forms as seen from the frontal plane (top), horizontal plane (middle) and sagittal plane (bottom) in LBBB without infarction (A), LBBB with anteroseptal infarction (B), LBBB with posterolateral infarction (C) and LBBB with inferior infarction (D). Colored lines represent areas of myocardium activated within the same 10-millisecond period (isochrones). Numbers represent milliseconds since beginning of activation. Key ECG changes include the development of large R waves in V1–V2 with anteroseptal infarction (B), increased R/R’ amplitude ratios in V5–V6 with apical infarction (B), increased S/S’ amplitude ratios in V1–V2 with posterolateral infarction (C) and Q waves and decreased R/Q or R/S in aVF amplitude ratios with inferior infarction (D).
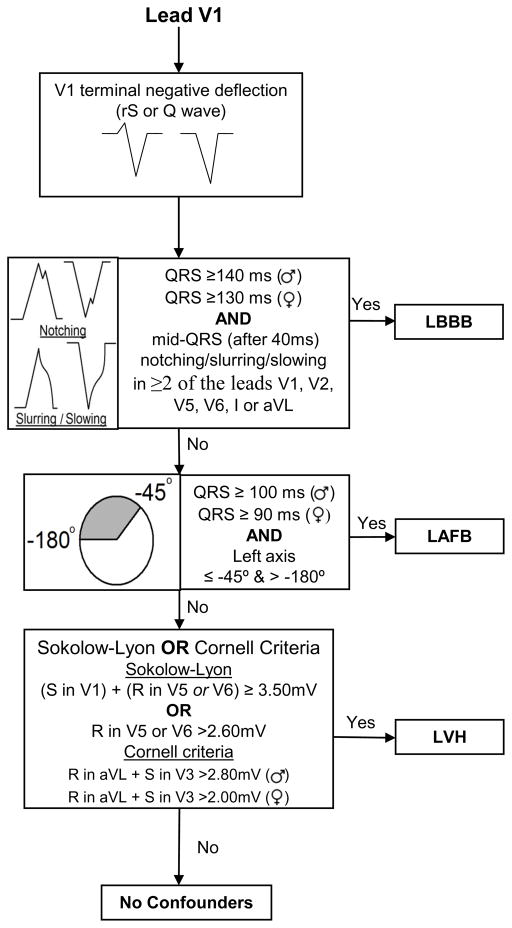
Figure 2. Flow chart for determining left conduction/hypertrophy type abnormality.
This chart describes the sequence in which ECGs are evaluated for conduction type when the terminal deflection in V1 is negative (rS or Q wave).
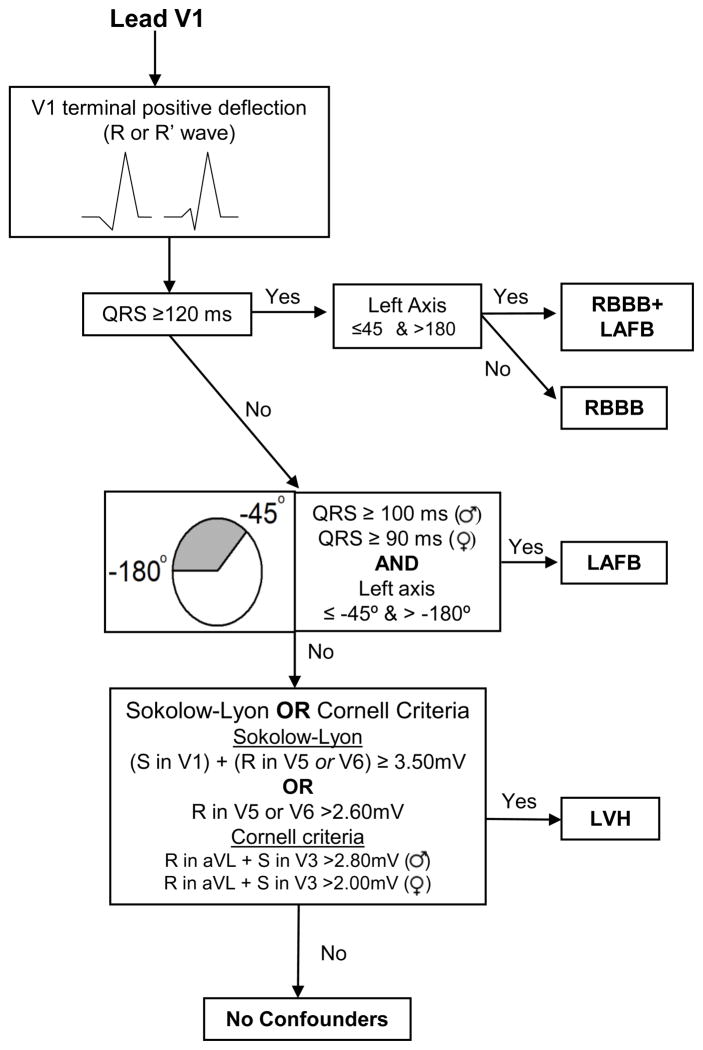
In RBBB, the RV activates via depolarization from the LV through the septum with the RV free wall activating last producing broad R or R’ waves in V1 and V2.18 This conduction pattern confounds the diagnosis of posterolateral infarcts which can also produce large R waves due to unopposed anterior forces. To account for this, in QRS scoring the R wave criteria in V1 and V2 for posterolateral scar are increased in the setting of RBBB and only the initial part of the R wave that peaks within the first 50 ms is considered (see Appendix 1). Furthermore, there are additional possible anterior points in the setting of RBBB to increase the sensitivity to large infarcts which are the most common cause of RBBB.8
Performing QRS Scoring
Classification of Conduction/ Hypertrophy Type
Figures 2 and 3 show flow diagrams that graphically represent the process of determining the QRS conduction/hypertrophy type “bin” in which to evaluate the ECG: LBBB, LAFB, LVH,RBBB+LAFB or no confounders. Lead V1 QRS morphology is considered to determine if there is a left versus right conduction/hypertrophy abnormality. If there is a terminal negative deflection in lead V1 (rS or Q morphology), then analysis proceeds according to the diagram in Figure 2 and if there is a terminal positive deflection in lead V1 (R or R’) then analysis proceeds according to the diagram in Figure 3.
Figure 3. Flow chart for determining left conduction/hypertrophy type abnormality.
This chart describes the sequence in which ECGs are evaluated for conduction type when the terminal deflection in V1 is positive (R or R’ wave).
As reported previously,2,8 the definitions for classifying LBBB and LAFB for QRS score purposes differ slightly from other reported definitions in the literature.19 The definitions used with QRS scoring correlate with the observations made from computer simulations of the different conduction types from which the QRS score criteria were derived.18,20 The critical difference with LBBB (recently described in detail21) is that the QRS scoring definition for LBBB requires the presence of mid-QRS notching and a minimum duration of 140 ms for men and 130 ms for women. To satisfy LAFB classification for QRS-scoring, we have used a minimum QRS duration of 100 ms because a block in the left anterior fascicle causes a prolongation of the QRS complex by 20 ms.22 However, because women have shorter baseline QRS duration then men,23 we propose a minimum QRS duration of 90 ms in women and 100 ms in men for defining LAFB. With regard to the lack of an upper boundary for QRS duration, LAFB may coexist with other QRS prolonging conduction defects such as LVH, a dilated ventricle or diffuse interstitial fibrosis leading to delayed intra-mural conduction. Patients meeting LAFB criteria with QRS duration greater than 120 ms undoubtedly have multiple pathologies (e.g. LAFB+LVH); however, these patients are most accurately evaluated by the LAFB QRS-score criteria. For the purposes of QRS scoring, we recommend using these definitions which were used during the development of QRS scoring,20 the validation in comparison to cardiac MRI2,3 and to predict outcomes from CRT and ICD therapy.15,16
For scoring purposes it does not matter if LVH accompanies one of the abnormal conduction types. The principal difference between QRS scores of LVH and no confounders is that in LVH, Q waves in V1 through V3 only receive QRS-score points if ≥ 4 QRS-score points are also present in leads I, aVL, V4, V5 or V6. This is because in LVH, Q waves may be present in leads V1, V2 and V3 in the absence of infarction; however evidence of infarction in other leads makes it more likely that the formation of Q waves was caused by an infarction. In addition, smooth Q waves can be normal in V1 – V3 in the presence of LVH, but notches in the initial 40ms (NtchInit40) are considered a sign of infarction. An ECG is considered to demonstrate LVH if either the Sokolow-Lyon or the Cornell criteria are satisfied (Figures 2 & 3).
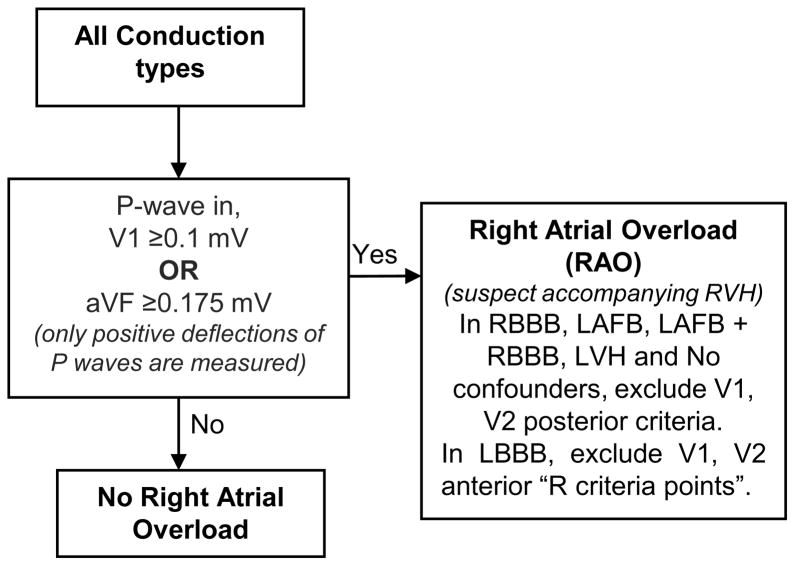
After determining conduction type, all ECGs should be evaluated for the presence of right atrial overload (RAO) by analysing the P waves in both leads V1 and aVF as shown in Figure 4. RAO commonly occurs in parallel with right ventricular hypertrophy (RVH).18,20 Large R waves in V1 and V2 are considered evidence of unopposed anterior electrical forces and are assigned points indicating infarction in the posterolateral wall of the LV or, in the case of LBBB, in the anteroseptal the wall; however, these R waves may also be due to increased anterior electrical force from RVH. If a patient has RAO, then large R waves in V1 and V2 are likely to be due to RVH rather than infarction.8
Figure 4. Flow chart for determining RAO.
After conduction type is determined by following Figure 2 or Figure 3, all ECGs are evaluated for presence of right atrial overload (RAO) which suggests accompanying right ventricular hypertrophy (RVH).
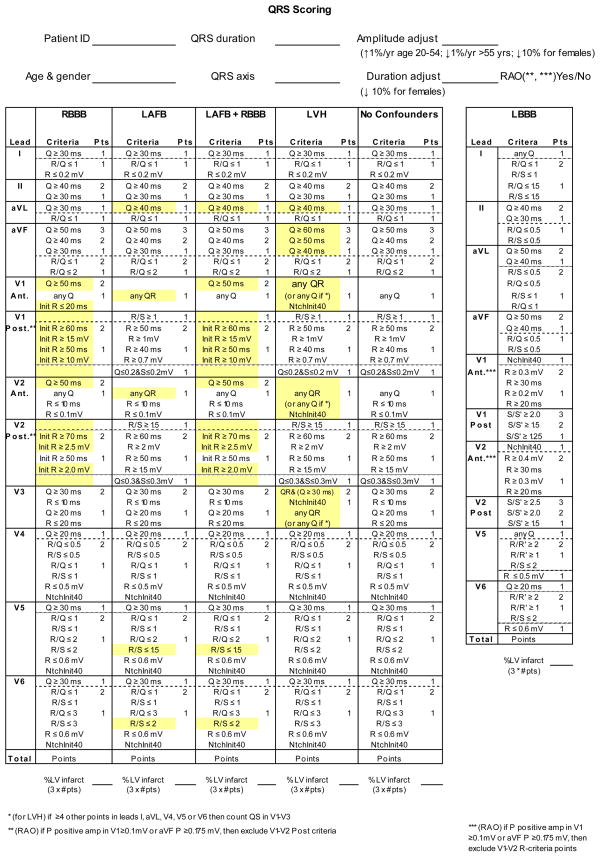
Leads V1 and V2 have different criteria for measuring anteroseptal and posterolateral scar. The QRS scoring sheet (Figure 5) lists the anteroseptal criteria as V1 anterior and V2 anterior in LBBB and simply as V1 and V2 in all other conduction types. Posterolateral criteria are listed as V1 posterior and V2 posterior in all conduction types. All ECGs should be analysed for the presence of both anteroseptal and posterolateral scar by evaluating both sets of criteria for leads V1 and V2. In the presence of RAO, we have excluded the V1 and V2 posterolateral QRS score criteria (anteroseptal criteria in LBBB) to prevent allocating false positive points due to RVH; however, they can be included in situations where sensitivity to detect scar is more valuable than specificity.
Figure 5. QRS Scoring Sheet.
Sample QRS scoring sheet with all conduction types and criteria listed. After demographic information is entered in the top portion, conduction type is selected and analysis proceeds down the appropriate column.
Application of QRS Scoring
False-positive points in QRS scoring were common in younger males who have increased voltage and older females who have lower voltages.8 Before scoring, all absolute amplitude criteria (not ratios) are corrected to the age of 55 in the scoresheet (Figure 5) by increasing them 1%/year for those aged 54 years and below and decreasing them 1%/year for those aged 56 years and above. For females, both duration and absolute amplitude (not ratio) criteria are further decreased by 10%.
After ECGs have been placed in conduction/ hypertrophy bins and amplitude and duration are corrected, the QRS score for that conduction/ hypertrophy type is selected and applied. Careful measurements of both amplitudes and durations are made with up to an 8× magnifier, when necessary.
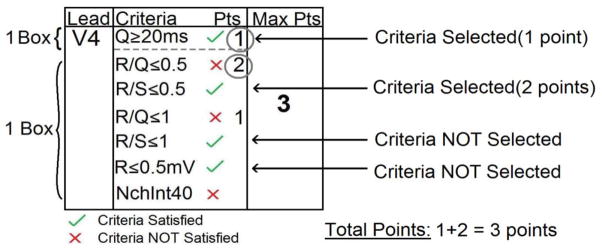
When scoring, two concepts must be considered: “weighting” and “selecting” (Figure 6). “Weighting” refers to the number of points awarded for the criteria satisfied which is provided to the right of the criteria. If the point value is not listed directly to the right of the criterion, its point value is the same as the criterion directly above it. “Selecting” is the process of choosing a single criterion from a group. When criteria with multiple weights are included within a box they are ordered by decreasing weights. Therefore, proceeding from the top down within a box (delineated by a dotted line), only the first satisfied criterion should be selected. Only one criterion can be selected for each box but there may be multiple boxes in each lead. The total point score for each ECG is the sum of the points accumulated from the criteria satisfied for each lead.
Figure 6. “Selecting” and “weighting” example.
Criteria for an example lead are shown. Boxes are delineated by dotted lines, check marks and X’s indicate which criteria are satisfied, and circles indicate criteria that are selected and contribute to the overall score for the lead. The Q ≥ 20 ms criterion is met yielding 1 point for the top box in this lead. Proceeding from the top down to the next box, the next criterion that is met is R/S ≤ 0.5 yielding 2 points (no point value is listed directly to the right of this criterion; therefore, its point value is equal to that of the criterion directly above it). Even though two additional criteria are met (R/S ≤ 1 and R ≤ 0.5 mV), only the single criterion within each box that is closest to the top of the box is selected.
Glossary and definitions
The glossary section in Appendices 1 and 2 contain definitions and illustrations of terms and criteria that are encountered when performing QRS scoring. The glossary is divided into two sections; the first is for no confounders, LVH, LAFB, RBBB and RBBB+LAFB conduction/ hypertrophy types (Appendix 1) and the second is only for LBBB conduction type (Appendix 2). This is due to LBBB having different criteria definitions compared to the other conduction/ hypertrophy types.
Examples
An example ECG with a completed score sheet is depicted in Figure 7. Additionally, the online supplement contains 26 example ECGs with both a blank test set and an annotated key with completed score sheets. This supplement is included to provide an opportunity to apply the QRS scoring criteria in the various conduction abnormalities on pre-scored examples.
Figure 7. QRS score example.
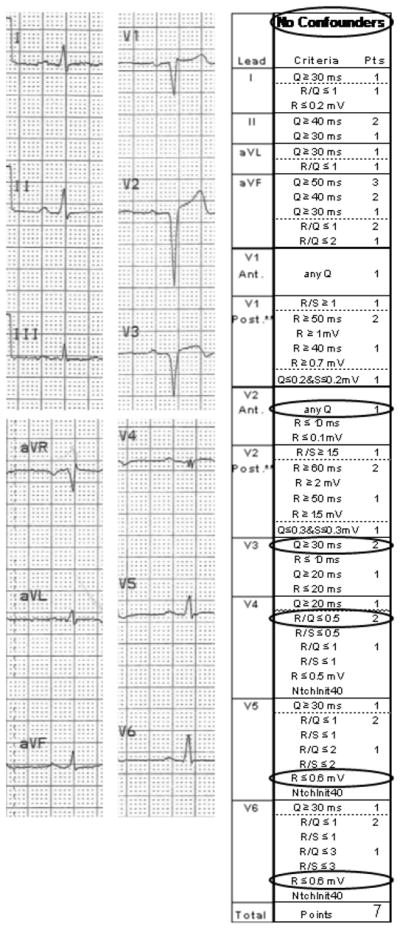
The ECG and completed QRS score sheet are shown for a 45 year old male with a QRS score of 7.
Limitations of QRS Scoring
As with all diagnostic modalities, the QRS score has limitations. Using P wave amplitude evidence of RAO to diagnose RVH is not a widely used method; however, it does not rely on features of the QRS complex which may be affected by infarction. Previous studies have found that P-wave analysis for diagnosing RVH has a sensitivity of 30–40% and a specificity of 80–91%.24,25 Because the presence of RAO is used to exclude evaluation of posterolateral infarction, specificity is more important than sensitivity. One consequence of this method is that patients with both RAO and posterolateral infarction (or anteroseptal infarction in LBBB) may have underestimated QRS scores. Another challenge arises when individual ECGs straddle the definitions of conduction/hypertrophy types. While the QRS-score criteria were tested when adhering to the previously outlined conduction/hypertrophy type definitions, further research may improve the classification of borderline ECGs, especially with regard to defining the presence vs. absence of LBBB.21
It is possible that beat-to-beat variation may lead to different numbers of points to be assigned to a lead depending on which beat is evaluated. The best way to limit bias and error in this setting is to score the most representative QRS complex (i.e. isoelectric baseline, no interceding premature beat etc.), or the computer-generated median beat, if available. Some of the criteria call for recognition of notches and/or slurs in the QRS complex. The presence and/or morphology of slurs and notches can be greatly affected by low ECG sampling rates or poor quality tracings. Sampling rates of at least 500 Hz are ideal for QRS scoring but not always available. Many of the previously cited studies utilized routinely acquired paper 12-lead ECGs suggesting that QRS scores derived from lower sample rate ECGs still provide clinically useful information. These studies demonstrated good intraobserver and interobserver agreement and manually applied QRS score criteria also demonstrated a strong ability to identify scar in reference to contrast-enhanced MRI (receiver operating characteristic area under the curve 0.91 [95% CI 0.86,0.95]) and good correlation with MRI scar size (r = 0.74, p < 0.0001).2,15 Limitations of beat-to-beat variability, poor-quality paper ECGs and the increasing complexity of the QRS score criteria can be improved by implementation of automated QRS-scoring algorithms.
Conclusions
The Selvester QRS score is a useful clinical tool that provides perspective on the relationship between cardiac electrical function and structural changes (namely scar presence and size). The clinical utility of understanding this relationship continues to be explored. By providing this detailed guide, we hope to empower others to continue to study QRS scoring, enable programming of automated QRS scoring and help determine how this understanding can best inform clinical decisions.
Supplementary Material
Two sets of ECGs are included that can be used to practice QRS scoring. ECG set 1 consists of 26 scanned ECGs along with a key with annotated ECGs and completed QRS scoring sheets. ECG set 2 contains 12 ECGs of higher quality along with a key with completed QRS scoring sheets.
Acknowledgments
Supported in part by Duke University's CTSA grant TL1RR024126 from NCRR/NIH (to Z. Loring) and FDA Critical Path and Office of Women’s Health grants (to D. Strauss).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selvester R, Wagner J, Rubin H. Quantitation of myocardial infarct size and location by electrocardiogram and vectorcardiogram. Quantitation in cardiology. 1972:31. [Google Scholar]
- 2.Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–36. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss DG, Cardoso S, Lima JA, Rochitte CE, Wu KC. ECG scar quantification correlates with cardiac magnetic resonance scar size and prognostic factors in Chagas' disease. Heart. 2011;97:357–61. doi: 10.1136/hrt.2010.210047. [DOI] [PubMed] [Google Scholar]
- 4.Wagner GS, Freye CJ, Palmeri ST, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. Circulation. 1982;65:342–7. doi: 10.1161/01.cir.65.2.342. [DOI] [PubMed] [Google Scholar]
- 5.Ideker RE, Wagner GS, Ruth WK, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. II. Correlation with quantitative anatomic findings for anterior infarcts. Am J Cardiol. 1982;49:1604–14. doi: 10.1016/0002-9149(82)90235-1. [DOI] [PubMed] [Google Scholar]
- 6.Roark SF, Ideker RE, Wagner GS, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. III. Correlation with quantitative anatomic findings for inferior infarcts. Am J Cardiol. 1983;51:382–9. doi: 10.1016/s0002-9149(83)80069-1. [DOI] [PubMed] [Google Scholar]
- 7.Ward RM, White RD, Ideker RE, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. IV. Correlation with quantitative anatomic findings for posterolateral infarcts. Am J Cardiol. 1984;53:706–14. doi: 10.1016/0002-9149(84)90390-4. [DOI] [PubMed] [Google Scholar]
- 8.Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Roubin GS, Shen WF, Kelly DT, Harris PJ. The QRS scoring system for estimating myocardial infarct size: clinical, angiographic and prognostic correlations. J Am Coll Cardiol. 1983;2:38–44. doi: 10.1016/s0735-1097(83)80374-x. [DOI] [PubMed] [Google Scholar]
- 10.Bounous EP, Jr, Califf RM, Harrell FE, Jr, et al. Prognostic value of the simplified Selvester QRS score in patients with coronary artery disease. J Am Coll Cardiol. 1988;11:35–41. doi: 10.1016/0735-1097(88)90163-5. [DOI] [PubMed] [Google Scholar]
- 11.Fioretti P, Tijssen JG, Azar AJ, et al. Prognostic value of predischarge 12 lead electrocardiogram after myocardial infarction compared with other routine clinical variables. Br Heart J. 1987;57:306–12. doi: 10.1136/hrt.57.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjandrawidjaja MC, Fu Y, Westerhout CM, Wagner GS, Granger CB, Armstrong PW. Usefulness of the QRS score as a strong prognostic marker in patients discharged after undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2010;106:630–4. doi: 10.1016/j.amjcard.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Richardson K, Engel G, Yamazaki T, Chun S, Froelicher VF. Electrocardiographic damage scores and cardiovascular mortality. Am Heart J. 2005;149:458–63. doi: 10.1016/j.ahj.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Barbagelata A, Califf RM, Sgarbossa EB, et al. Prognostic value of predischarge electrocardiographic measurement of infarct size after thrombolysis: insights from GUSTO I Economics and Quality of Life substudy. Am Heart J. 2004;148:795–802. doi: 10.1016/j.ahj.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Strauss DG, Poole JE, Wagner GS, et al. An ECG index of myocardial scar enhances prediction of defibrillator shocks: an analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2011;8:38–45. doi: 10.1016/j.hrthm.2010.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010;121:626–34. doi: 10.1161/CIRCULATIONAHA.109.894774. [DOI] [PubMed] [Google Scholar]
- 17.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 18.Selvester RH, Wagner GS, Ideker RE. Myocardial Infarction. In: Macfarlane PW, Lawrie TDV, editors. Comprehensive electrocardiology: theory and pracice in health and disease. New York: Pergammon Press; 1989. p. 565. [Google Scholar]
- 19.Surawicz B, Childers R, Deal BJ, Gettes LS. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part III: Intraventricular Conduction Disturbances A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology. 2009;53:976. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Selvester RH, Strauss DG, Wagner GS. Myocardial Infarction. In: Macfarlane PW, Van Oosterom A, Pahlm O, Kligfield P, Janse M, Camm J, editors. Comprehensive Electrocardiology. London: Springer-Verlag; 2011. pp. 651–746. [Google Scholar]
- 21.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum MB, Elizari MV, Levi RJ, et al. Five cases of intermittent left anterior hemiblock. The American Journal of Cardiology. 1969;24:1–7. doi: 10.1016/0002-9149(69)90044-7. [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane P, Lawrie TDV, editors. Comprehensive Electrocardiology. New York: Pergamon; 2011. pp. 2057–2155. Appendix 1: Adult Normal Limits. [Google Scholar]
- 24.Lewczuk J, Ajlan AW, Piszko P, et al. Electrocardiographic signs of right ventricular overload useful in improving diagnosis of chronic thromboembolic pulmonary hypertension (CTE-PH) in patients with chronic exertional dyspnea. Pol Arch Med Wewn. 2002;108:1049–54. [PubMed] [Google Scholar]
- 25.Henkens IR, Mouchaers KT, Vonk-Noordegraaf A, et al. Improved ECG detection of presence and severity of right ventricular pressure load validated with cardiac magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2008;294:H2150–7. doi: 10.1152/ajpheart.01312.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two sets of ECGs are included that can be used to practice QRS scoring. ECG set 1 consists of 26 scanned ECGs along with a key with annotated ECGs and completed QRS scoring sheets. ECG set 2 contains 12 ECGs of higher quality along with a key with completed QRS scoring sheets.