Table 2.
Anti-proliferative activity of cyclohexyl analogues.
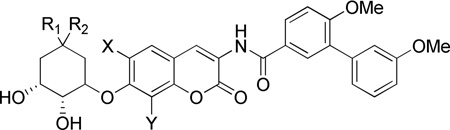 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound (IC50, µM) |
R1 | R2 | X | Y | SKBr3 | MCF-7 | LNCAP-LN3 | PC3-MM2 |
| 43a | CH3 | CH3 | H | CH3 | >100a | >100a | 1.24 ± 0.17b,d | 2.49 ± 1.70b |
| 43b | CH3 | CH3 | H | OCH3 | 3.45 ± 1.73 | 1.56 ± 0.03 | NT | NT |
| 43c | CH3 | CH3 | OCH3 | CH3 | >100 | >100 | NT | NT |
| 44a | H | CH3 | H | CH3 | >100 | >100 | 1.14 ± 0.67 | 4.09 ± 1.63 |
| 44b | H | CH3 | H | OCH3 | 6.38 ± 0.71 | 8.52 ± 0.36 | NT | NT |
| 44c | H | CH3 | OCH3 | CH3 | >100 | >100 | NT | NT |
| 4541 | H | H | H | CH3 | >100a | >100 | 1.58 ± 0.75 | 4.04 ± 0.38d |
| 45b | H | H | H | OCH3 | 7.44 ± 0.36 | 5.46 ± 0.36 | NT | NT |
| 45c | H | H | OCH3 | CH3 | 8.18 ± 0.79 | 10.13± 1.04 | NT | NT |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate.
Values represent mean ± standard deviation from dose response curves for at least two separate experiments performed in duplicate.
