Table 6.
Anti-proliferative activity of optimized analogues.
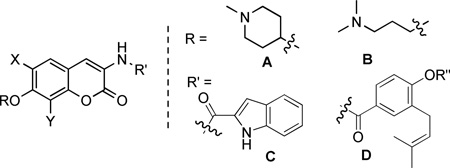 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound (IC50, µM) |
R | X | Y | R’ | R” | SKBr3 | MCF-7 | LNCAP-LN3 | PC3-MM2 |
| 78a | A | H | CH3 | C | -- | 0.48 ± 0.09a | 0.57 ± 0.03a | 11.83 ± 0.54b | 11.40 ± 5.25b |
| 78b | A | H | OCH3 | C | -- | 2.58 ± 0.28 | 1.86 ± 0.08 | 12.64 ± 0.32d | 7.93 ± 4.18 |
| 78c | A | OCH3 | CH3 | C | -- | 0.11 ± 0.01 | 0.52 ± 0.04 | 1.47 ± 0.53 | 0.87 ± 0.46 |
| 79a | A | H | CH3 | D | Ac | 0.58 ± 0.04 | 1.18 ± 0.16 | NT | 2.12 ± 3.32 |
| 79b | A | H | OCH3 | D | Ac | 1.07 ± 0.14 | 1.64 ± 0.24 | NT | 3.98 ± 0.06d |
| 79c | A | OCH3 | CH3 | D | Ac | 0.42 ± 0.01 | 0.58 ± 0.02 | NT | 1.41 ± 0.04d |
| 80a | A | H | CH3 | D | H | 0.76 ± 0.14 | 1.09 ± 0.08 | NT | 1.37 ± 1.42 |
| 80b | A | H | OCH3 | D | H | 0.92 ± 0.01 | 1.54 ± 0.21 | NT | 3.53 ± 0.01d |
| 80c | A | OCH3 | CH3 | D | H | 0.42 ± 0.01 | 0.54 ± 0.02 | NT | 2.26 ± 1.43 |
| 82a | B | H | CH3 | C | -- | 1.13 ± 0.01 | 5.23 ± 0.22 | NT | 13.69 ± 0.18d |
| 82b | B | H | OCH3 | C | -- | 1.50 ± 0.13 | 1.41 ± 0.09 | 4.71 ± 1.23d | 8.95 ± 6.11 |
| 82c | B | OCH3 | CH3 | C | -- | 0.57 ± 0.09 | 0.56 | NT | 2.58 ± 4.47 |
| 83a | B | H | CH3 | D | Ac | 0.46 ± 0.15 | 1.18 ± 0.02 | NT | 1.42 ± 0.05d |
| 83b | B | H | OCH3 | D | Ac | 0.78 ± 0.17 | 2.14 ± 0.22 | NT | 4.59 ± 4.23 |
| 83c | B | OCH3 | CH3 | D | Ac | 0.36 ± 0.03 | 0.70 ± 0.03 | NT | 1.46 ± 0.03d |
| 84a | B | H | CH3 | D | H | 0.44 ± 0.02 | 1.35 ± 0.30 | NT | 1.81 ± 1.22 |
| 84b | B | H | OCH3 | D | H | 0.77 ± 0.08 | 3.26 ± 0.26 | NT | 9.24 ± 17.79 |
| 84c | B | OCH3 | CH3 | D | H | 0.39 ± 0.06 | 0.80 ± 0.07 | NT | 1.38 ± 0.02d |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate.
Values represent mean ± standard deviation from dose response curves for at least two separate experiments performed in duplicate.
