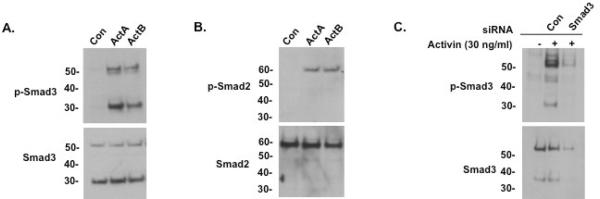
Fig. 3.
Characterization of the Smad3 isoform protein in LβT2 cells. (A) Immunoblots of vehicle (Con), activin A (30 ng/ml), or activin B (30 ng/ml)-treated LβT2 cell lysates, using either anti-phospho-Smad3 antibody (top) or anti-Smad3 antibody (bottom). Both of activin A and B treatment induced a full-length phospho-Smad3 (52 kDa) and a phospho-Smad3 isoform protein (about 30 kDa). Unphosphorylated full-length and Smad3 isoform proteins were also present in untreated cells including activin-treated cells. (B) Immunoblots of vehicle (Con), activin A or activin B (30 ng/ml)-treated LβT2 cell lysates, using either anti-phospho-Smad2 antibody (top) or anti-Smad2 antibody (bottom). A single form of phospho-Smad2 (60 kDa) was seen in LβT2 cells upon treatment with activin A or activin B; unphosphorylated Smad2 was detected in untreated cells. (C) Anti-phospho-Smad3 (top) and anti-Smad3 (bottom) immunoblots of LβT2 cells transfected with 100 nM of control (Con) or mouse Smad3-specific siRNA oligoduplexes per well using Lipofectamine 2000, then treated with activin A (30 ng/ml) for 1 hour. Both full-length and small Smad3 and phospho-Smad3 proteins were knocked down by Smad3-specific siRNA.