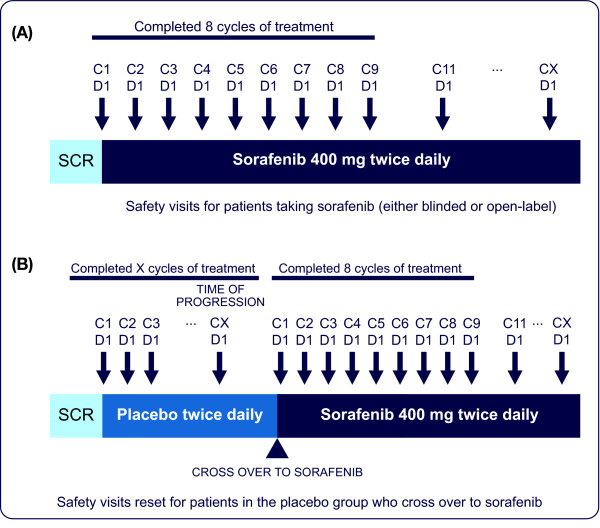
Figure 3.
Safety assessments. (A) Patients treated with sorafenib during the double-blind treatment period and those treated with sorafenib who experience PD before completing 8 cycles and are unblinded (open-label treatment period). (B) Patients who received placebo during the double-blind treatment period and then crossed over to sorafenib after unblinding.