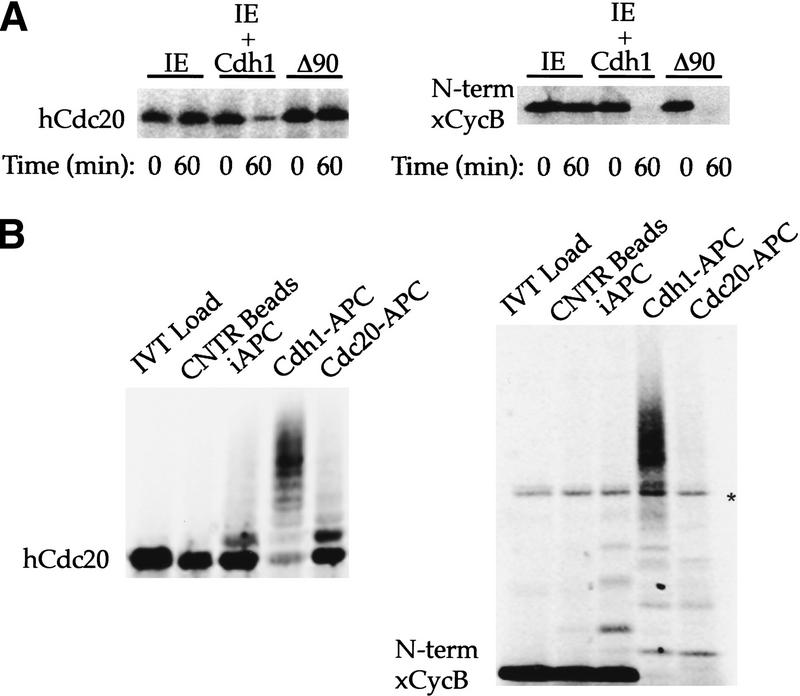
Figure 1.
Recognition of Cdc20 as a substrate by Cdh1–APC but not Cdc20–APC. (A) Incubation of 35S-labeled in vitro-translated hCdc20 and the amino terminus of Xenopus cyclin B (xCycB) in interphase extract supplemented with buffer (IE), interphase extract supplemented with Cdh1 protein (IE + Cdh1), or interphase extract pushed into mitosis by sea urchin cyclin B Δ90 (Δ90). Both hCdc20 and xCycB are stable in IE, but degrade rapidly in IE + Cdh1. xCycB, and not hCdc20, disappears in the mitotic extract. (B) Incubation of hCdc20 and amino-terminal xCycB in a purified system ubiquitination assay using control beads (Cntr Beads), immunopurified interphase APC on beads (iAPC), or iAPC on beads activated by Cdh1 (Cdh1–APC) or Cdc20 (Cdc20–APC). Neither substrate forms high molecular weight conjugates in the presence of control beads or iAPC, but both form significant higher molecular weight conjugates in the presence of Cdh1–APC. Only xCycB forms higher molecular weight conjugates in the presence of Cdc20–APC. Polyubiquitinated species of xCyc B spread out more in the presence of Cdc20–APC than in the presence of Cdh1–APC, creating a much lighter smearing not always easily visualized for the 35S-labeled cyclin fragment as with iodinated substrate in previous reports. A darker exposure (not shown) makes visualization of the conjugates more obvious. An asterisk indicates the presence of a background band present in the in vitro translation products.