Abstract
PCOS appears to be an ancient disorder, which persisted in human evolution despite reduced fecundity because of benefits to affected women such as greater sturdiness and improved energy utilization, a rearing advantage for their children and kin, and a reduction in the risk of perinatal mortality. This raises the possibility that gene variants eventually found to be associated with PCOS will be similar across ethnic groups and races.
Keywords: PCOS, evolution, susceptibility genes
POLYCYSTIC OVARY SYNDROME: AN ANCIENT DISORDER?
And all the days of Methuselah were nine hundred sixty and nine years: and he died. Genesis 5:27
Is Polycystic Ovary Syndrome (PCOS) an ancient disorder? Or is it a disorder of recent development, the consequence of rising metabolic stress in an increasingly obese society? And if it is ancient, why has it persisted despite its reproductive disadvantage? And can the antiquity and evolutionary history of PCOS inform our efforts to unravel its genetic makeup?
Ancient medical records
While there is little in the Egyptian papyri (Kahun, Edwin Smith, and Ebers) regarding the antiquity of PCOS, an examination of later ancient medical records provides clues. Hippocrates (460 BC-377 BC) notes that “But those women whose menstruation is less than three days or is meagre, are robust, with a healthy complexion and a masculine appearance; yet they are not concerned about bearing children nor do they become pregnant.” (Diseases of Women 1.6) (1). Soranus of Ephesus (c. 98-138 AD) noted that “[s]ometimes it is also natural not to menstruate at all... It is natural too in persons whose bodies are of a masculine type... we observe that the majority of those not menstruating are rather robust, like mannish and sterile women” (Gynecology, Book I. Art. 23 and Book I. Art. 29) (2).
The medieval physician Moises Maimonides (1135-1204 AD) noted that “...there are women whose skin is dry and hard, and whose nature resembles the nature of a man. However, if any woman's nature tends to be transformed to the nature of a man, this does not arise from medications, but is caused by heavy menstrual activity” (Fin Liber Comm. Epidemirum VI, 8) (3). Most directly, the celebrated renaissance surgeon and obstetrician Ambroise Pare (1510-1590 AD) observed that “Many women, when their flowers or tearmes be stopped, degenerate after a manner into a certaine manly nature, whence they are called Viragines, that is to say stout, or manly women; therefore their voice is loud and bigge, like unto a mans, and they become bearded.” (The 24th Book-Of the Generation Of Man) (4).
These statements made over a period of more than two millennia describe a combination of signs, including menstrual irregularity, masculine habitus, sub-infertility, and possible obesity, suggestive of PCOS. They also describe the disorder in terms that translate today as ‚sometimes’ or ‚many’, indicating the condition was sufficiently common to merit description.
The racial prevalence of PCOS
An examination of the global prevalence of PCOS today may be instructive in elucidating differences between the races. Reports from the United States, the United Kingdom, Spain, Greece, Australia, and Mexico demonstrate a strikingly similar prevalence of PCOS, as defined by the NIH 1990 criteria, ranging from 6-9% (5-10). While many populations remain to be studied and most of the subjects in these reports were Whites of European descent, the African-American (6) and Mexican (10) women included also demonstrated similar prevalences. Consequently, considering that humans migrated from Africa by 50,000 years ago (11), the PCOS genotype(s) appears to have emerged earlier than the onset of racial diversity.
Is the prevalence of PCOS affected by the increasing rates of obesity?
The prevalence of PCOS appears to be only minimally affected by the increasing rates of obesity and the excessive consumption of Western-type food. For example, the prevalence of PCOS is relatively similar across countries with different rates of obesity (e.g. United States vs. Spain or Mexico) (5, 6, 10). Likewise, we have been unable to detect significant differences in the dietary intake or composition of women with PCOS compared to matched controls (12). In a study of over 600 unselected women from the general population, the prevalence of PCOS increased minimally and non-significantly with increasing body mass (13). In contrast, the average body mass of over 700 women with PCOS diagnosed over a 15-year interval increased linearly and in concert with the increasing obesity of the surrounding population (13). Taken together, these data suggest that the epidemics of excess caloric intake and overweightness play a limited role in the development of PCOS.
But why should a disorder that reduces reproductive potential thrive and persist across millennia?
It is likely that, as for other susceptibility alleles for modern metabolic diseases (14, 15), the origins of PCOS began in Paleolithic hunter-gatherer communities, in which environmental stressors favored the survival of those males, females and offspring with the greatest capacity for energy storage necessary to endure prolonged episodes of privation, the so-called “thrifty genotype” (16-18). If so, such a thrifty genotype may have enhanced survival during times of food deprivation, with reduced postprandial thermogenesis from insulin resistance diminishing energy expenditure as an additional evolutionary advantage (19).
Furthermore, we should note that few women with PCOS are actually sterile and PCOS family size can be normal in today's society of medical therapy and family-planning (20), proof that conditions restricting reproduction, whether social or environmental, ameliorate the reproductive disadvantage to PCOS families. Moreover, in a study of over 300 women with PCOS, those women treated with placebo ovulated approximately one-third the expected (monthly) frequency (21); thus, PCOS women are able to conceive, albeit at a rate lower than normal. Considering the reproductive benefits of coitus initiation at an early age, a higher coital frequency, the absence of effective contraceptives, and the absence of widespread obesity, it is quite possible that the pregnancy rates of ancient PCOS women would have been significantly higher than at present, particularly if relative insulin resistance was able to divert circulating glucose as dietary energy for ovulatory function during low energy conditions (22).
In addition, among nomadic hunters it would have been advantageous and even necessary for women to space childbirth, as they could generally carry and care for only one young child at a time (23). Childbirth-related complications were an important cause of mortality in reproductive-aged women in antiquity (and in present-day Africa), and a lower parity may have reduced the death rate of these women and the risk of progeny abandonment. The lower fecundity of PCOS women could also have created a rearing advantage for their progeny, with their fewer children receiving a greater amount of the available food and protection. And these progeny, both as a result of their inherited genotype, and possibly the effect of their intrauterine environment, would have also been more able to survive periodic deprivation.
It could also be argued that PCOS favored the survival of those family units containing these women, as females with PCOS and few, if any, children of their own could have served as allo-mothers to their kin (24). With aging, PCOS women may have attained significant nurturing skills, given their wisdom and strength to survive a physically demanding environment, creating a source of capable child rearing labor not focused on or threatened by pregnancy. Finally, in such a physically demanding environment, the greater lean muscle mass and bone mineral density of PCOS women (25-28) would have also been advantageous to their own survival and that of their progeny. Thus, the disease susceptibility alleles for PCOS may have been ideally adapted to the need for high physical strength and activity, the erratic and often low nutrient availability, and the lower fecundity advantageous to the hunter-gatherer.
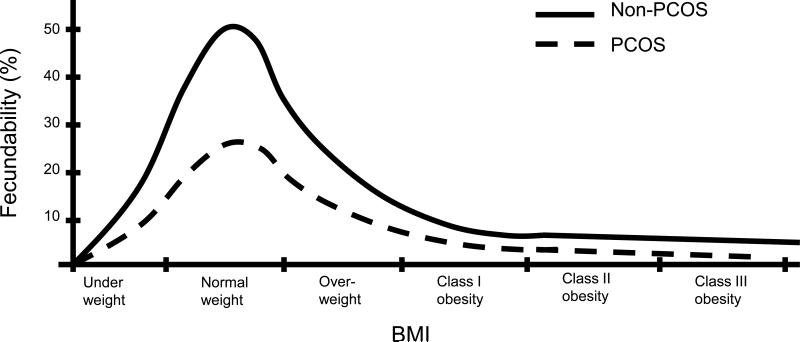
In a recent interesting analysis, Corbett et al (29) propose that the BMI-fecundity relationship of PCOS is simply shifted leftward, such that patients with PCOS would have „normal’ fertility at the very low BMIs experienced by hunter-gatherers (and including populations through the 1800s) during periods of food scarcity, while their non-PCOS counterparts would experience subnormal fertility during that period of time. In contrast, and based on our observations of BMI and fertility in PCOS (6, 13), we suggest that the BMI-fecundity relationship in PCOS is simply decreased at all BMIs (see Figure 1). Thus, patients with PCOS should be considered subfertile, not infertile. In our model, the persistent reproductive capacity of PCOS women in antiquity does not rely on a shifting of the BMI-fecundity curve, but on: 1) the BMIs in general being lower in the pre-1800s, and thus the lessening of the rate of subfertility in PCOS, although not fully approaching the fertility of non-PCOS (i.e. normal) women; and 2) the reduced obstetrical morbidity/mortality associated with the subfertility.
Figure 1.
Proposed BMI-fecundity relationship in PCOS
Why did the PCOS genotypes survive the Neolithic revolution?
If the PCOS alleles developed, as other metabolic complex traits appear to have done, in the hunter-gatherer Paleolithic period of the Stone Age, why did these genotypes survive the Neolithic revolution of some 10,000 years ago, with its adoption of farming, animal domestication, and sedentary settlements? In fact, ancestral genetic traits determining modern metabolic diseases, such as cardiovascular disease and diabetes, did persist in sedentary societies, and were evident even in ancient Egypt (c. 1500 BC) (30, 31). But these disorders generally would have affected individuals who were older (30) and would have had little impact on the reproductive potential of the affected individuals, perhaps being of limited selective importance in a society whose average life-span was approximately 35 years.
In contrast with the hunter-gatherer society, having larger numbers of children was a necessity for the survival of agricultural communities to provide the necessary labor while overcoming the high neonatal mortality evident at the time (32). In an agricultural environment, in which sufficient food eventually became available, PCOS should have been an adaptive disadvantage to any affected female, and might have been eliminated over the past 10,000 years, consistent with the rapid evolution observed for other traits (33). This hypothesis, however, assumes that the reproductive disadvantage of PCOS is absolute.
However, as for hunter-gatherers, the reproductive disadvantages of PCOS women in the Neolithic and subsequent periods would have been less severe than currently presumed. Like hunter-gatherer females, PCOS women in sedentary agricultural societies may have been able to conceive, albeit at a rate lower than normal, may have had lower maternal mortality, would have been able to serve as allo-mothers to their kin, and would have been sturdier than average; their progeny may have had the advantage of their genotype, their intrauterine environment, and the increased maternal attention and protection. Furthermore, significant periodic privations remained a fact of life through the late 1700s and early 1800’s, for which women with PCOS are better suited to survive (29).
Finally, affected male relatives, while demonstrating evidence of androgen excess (34) and insulin resistance (35), do not demonstrate obvious impairment in fertility or ability to attract a suitable partner and in ancient times would even have had the metabolic advantages of PCOS females, and thus were readily able to transmit susceptibility alleles. Consequently, the metabolic and physical advantages of affected women and their progeny, the rearing advantages of their family units, the heterogeneity of the phenotype and genotype, and ready transmissibility of the genotype by affected males could have potentially equalized any disadvantages arising from the overall lower number of children begotten by PCOS women.
Can the antiquity of PCOS inform our efforts to identify susceptibility genes?
PCOS is likely the result of a myriad of genetic variations resulting in a complex genetic trait. In fact, mathematical modeling has suggested that Mendelian-disease genes appear to be under widespread purifying selection, especially when the disease mutations are dominant (rather than recessive); in contrast, the class of genes that influence complex disease risk show little signs of negative selection, possibly because this category includes targets of both purifying and positive selection (14). If PCOS were indeed ancient, we would expect that the susceptibility genes would in great measure be shared between different ethnic groups.
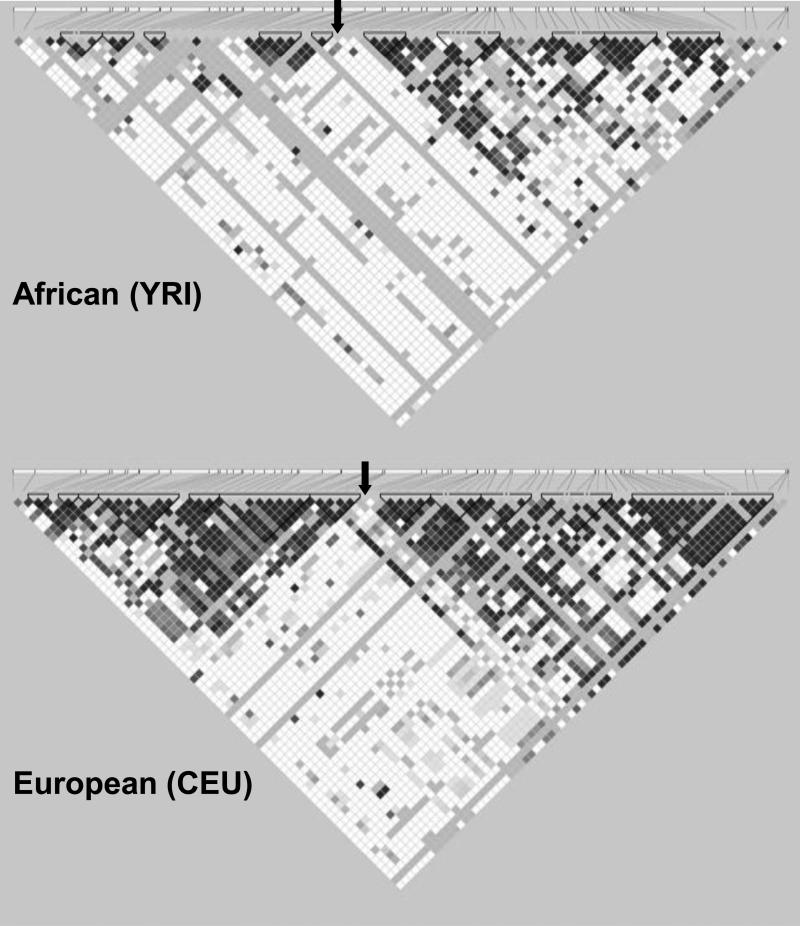
A potential challenge in identifying genes for an ancient disorder is that the linkage disequilibrium (LD) surrounding susceptibility loci may be relatively restricted, as predisposing loci arising in ancient times would be more separated from neighboring markers by recombination. This is suggested by the fact that older population groups (e.g. Africans) have less extensive LD blocks than more recent populations (e.g. Europeans) (36) (Figure 2). Limited LD would necessitate very dense (e.g. 1 million or greater) sets of markers to identify these loci. It remains to be seen whether such considerations hinder gene discovery in genome-wide association studies (GWAS) of PCOS currently underway, and highlights the potential utility of candidate gene studies in PCOS, wherein targeted genetic regions are readily covered at much greater density than by GWAS.
Figure 2.
Effect of time on linkage disequilibrium. Both linkage disequilibrium (LD) plots represent a 100-kb area centered around the microsatellite D19S884 (location approximately indicated by black arrows), a marker for which one allele has been associated with PCOS in multiple studies (37). The data were downloaded from the International HapMap Project (Phase II+III, Release 27, http://hapmap.ncbi.nlm.nih.gov); plots were generated using Haploview (version 4.2, http://www.broadinstitute.org/mpg/haploview). The top LD plot is from Yoruba subjects from Ibadan, Nigeria (YRI), and the bottom panel is that of the Utah residents with ancestry from northern and western Europe (CEU). The LD plots display D’ values for each pair of SNPs in the box at the intersection of the diagonals from each SNP. The darker boxes indicate greater degrees of LD for the corresponding pair of variants. Triangles around boxes indicate haplotype blocks determined by the confidence intervals algorithm in Haploview. The older Yoruba population exhibits less extensive LD and smaller haplotype blocks.
It is difficult to predict what effect the ancientness of PCOS would have on the effect sizes of susceptibility alleles. Purifying selection against alleles with severe consequences may have resulted in a set of remaining susceptibility loci with mild effects on PCOS risk. Alternatively, balanced selection due to the selective advantages described above could have preserved susceptibility alleles with more substantial effect sizes. If GWAS do identify PCOS susceptibility loci, an examination of whether such loci manifest evidence of positive selection would provide genetic support to our hypothesis that PCOS persisted through the ages by conferring a survival advantage.
Conclusions
Taken together, these observations suggest that PCOS is an ancient disorder, arising from ancestral gene variants selected during the Paleolithic Period and maintained over the past 10,000 years following the onset of Neolithic culture. Such ancient genes were likely transmitted transgenerationally through offspring conceived between fertile carrier males and subfertile affected females; the reduced fecundity of affected women potentially would have been offset, at least in part, by their greater sturdiness and improved energy utilization, a rearing advantage for their children and kin, and a reduction in the risk of maternal mortality. While this analysis cannot allow us to determine whether the gene variants eventually found to be associated with PCOS will be similar across ethnic groups and races, it does raises the possibility that a dense set of markers will be required to elucidate them in genome-wide approaches.
Acknowledgements
The authors would like to thank Dr. Jerome I. Rotter, for his insight and helpful comments.
Financial Support: This study was supported in part by NIH grants R01-HD29364 (to RA), R01-DK79888 (to M.O.G.), the Winnick Clinical Scholars Award (to M.O.G.), and an endowment from the Helping Hand of Los Angeles, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: M.O.G., D.A.D., and R.A. have nothing to declare.
Capsule: Polycystic ovary syndrome appears to be an ancient disorder, such that susceptibility loci for PCOS may be shared among diverse populations.
REFERENCES
- 1.Hanson AE. Hippocrates: Diseases of Women 1. Signs (Chic) 1975;1:567–84. doi: 10.1086/493243. [DOI] [PubMed] [Google Scholar]
- 2.Temkin O. Soranus’ Gynecology. The Johns Hopkins University Press; Baltimore: 1991. [Google Scholar]
- 3.Rosner F, Munter S. The Medical Aphorism of Moses Maimonides, Vol. II. Yeshiva University Press; New York: 1971. [Google Scholar]
- 4.Paré A. The causes of the suppression of the courses or menstrual fluxe. Chap. LI, Lib. 24. In: Johnson T, editor. The Workes of that famous Chirurgion Ambrole Parey: Translated out of Latine and compared with the French. Th. Cotes and R. Young; London: 1634. p. 947. [Google Scholar]
- 5.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 8.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2009 Nov 12; doi: 10.1093/humrep/dep399. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf) 1999;51:779–86. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 10.Moran C, Tena G, Moran S, Ruiz P, Reyna R, X. D. Prevalence of polycystic ovary syndrome and related disorders in Mexican women. Gynecol Obstet Invest. 2010;69:274–80. doi: 10.1159/000277640. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Prugnolle F, Manica A, Balloux F. A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet. 2006;79:230–7. doi: 10.1086/505436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril. 2006;86:411–7. doi: 10.1016/j.fertnstert.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162–8. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blekhman R, Man O, Herrmann L, Boyko AR, Indap A, Kosiol C, et al. Natural selection on genes that underlie human disease susceptibility. Curr Biol. 2008;18:883–9. doi: 10.1016/j.cub.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosiol C, Vinar T, da Fonseca RR, Hubisz MJ, Bustamante CD, Nielsen R, et al. Patterns of positive selection in six Mammalian genomes. PLoS Genet. 2008;4:e1000144. doi: 10.1371/journal.pgen.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–8. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 18.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 1992;36:537–43. doi: 10.1111/j.1365-2265.1992.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 20.Pall M, Stephens K, Azziz R. Family size in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1837–9. doi: 10.1016/j.fertnstert.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626–32. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 22.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–33. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 23.Diamond J. Guns, germs, and steel. WW Norton and Company; New York: 1999. [Google Scholar]
- 24.Eggers S, Hashimoto DM, Kirchengast S. Anthropologischer Anzeiger; Bericht über die biologisch-anthropologische. Literatur. 2007;65:169–79. [PubMed] [Google Scholar]
- 25.Carmina E, Guastella E, Longo RA, Rini GB, Lobo RA. Correlates of increased lean muscle mass in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:583–9. doi: 10.1530/EJE-09-0398. [DOI] [PubMed] [Google Scholar]
- 26.Di Carlo C, Shoham Z, MacDougall J, Patel A, Hall ML, Jacobs HS. Polycystic ovaries as a relative protective factor for bone mineral loss in young women with amenorrhea. Fertil Steril. 1992;57:314–9. doi: 10.1016/s0015-0282(16)54837-7. [DOI] [PubMed] [Google Scholar]
- 27.Good C, Tulchinsky M, Mauger D, Demers LM, Legro RS. Bone mineral density and body composition in lean women with polycystic ovary syndrome. Fertil Steril. 1999;72:21–5. doi: 10.1016/s0015-0282(99)00203-4. [DOI] [PubMed] [Google Scholar]
- 28.To WW, Wong MW. A comparison of bone mineral density in oligomenorrhoeic adolescents with polycystic ovaries and normal ovaries. Gynecol Endocrinol. 2005;20:237–42. doi: 10.1080/09513590500097200. [DOI] [PubMed] [Google Scholar]
- 29.Corbett SJ, McMichael AJ, Prentice AM. Type 2 diabetes, cardiovascular disease, and the evolutionary paradox of the polycystic ovary syndrome: a fertility first hypothesis. Am J Hum Biol. 2009;21:587–98. doi: 10.1002/ajhb.20937. [DOI] [PubMed] [Google Scholar]
- 30.Allam AH, Thompson RC, Wann LS, Miyamoto MI, Thomas GS. Computed tomographic assessment of atherosclerosis in ancient Egyptian mummies. Jama. 2009;302:2091–4. doi: 10.1001/jama.2009.1641. [DOI] [PubMed] [Google Scholar]
- 31.Christopoulou-Aletra H, Papavramidou N. 'Diabetes’ as described by Byzantine writers from the fourth to the ninth century AD: the Graeco-Roman influence. Diabetologia. 2008;51:892–6. doi: 10.1007/s00125-008-0981-4. [DOI] [PubMed] [Google Scholar]
- 32.Gurven M, Kaplan H. Longevity among hunter-gatherers: a cross-cultural examination. Popul Dev Rev. 2007;33:321–65. [Google Scholar]
- 33.Cochran G, Harpending H. The 10,000 Year Explosion: How Civilization Accelerated Human Evolution. Basic Books; New York: 2009. [Google Scholar]
- 34.Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:2134–8. doi: 10.1210/jcem.87.5.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–6. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 37.Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, et al. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–7. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]