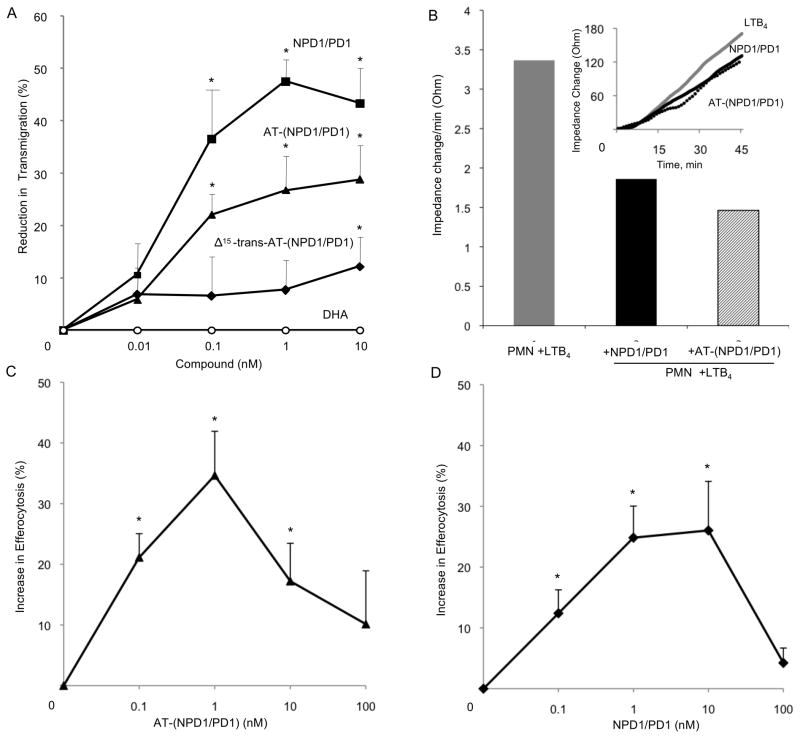
Figure 6. AT-(NPD1/PD1) limits human PMN transmigration across human endothelial cells and enhances human macrophage efferocytosis of apoptotic human PMNs.
Panel A: Neutrophils (106 cells per monolayer) were exposed to vehicle containing buffer or indicated concentrations of NPD1/PD1 (square), AT-(NPD1/PD1) (triangle), Δ15-trans-AT-NPD1/AT-PD1 (diamond) or DHA (circle) (15 min, pH 7.45, PBS-/-, 37ºC). Transmigration was initiated with addition of 10-8 M LTB4 (90 min, 37ºC). Results are mean ±SEM obtained from 3-5 separate PMN donors, each point in triplicate. Panel B: HUVECs were plated (0.2×106/well) in an ECIS chamber for 24 h. After 24 h, PMN were isolated from human donors and incubated with AT- (NPD1/PD1) or NPD1/PD1 (1 nM) for 15 min, 37ºC. LTB4 (10nM) and PMN (106 cells) were then added to the HUVECS. Impedance changes were monitored using Applied Biophysics ECIS software. (B) Representative real time tracing of impedance changes. Results are mean ± S.E.M. of n=3 separate donors and HUVEC preparations, *p<0.05. Using a two-way ANOVA, NPD1/PD1 was not significantly different from AT-NPD1/PD1 in reducing PMN transendothelial migration, P > 0.05.
Panels C and D: Human macrophages (105 per well) were exposed to vehicle containing buffer or indicated concentrations of AT-(NPD1/PD1) (black triangles) or NPD1/PD1 (black squares) (15 min, pH 7.45, PBS -/-, 37ºC). Uptake was initiated with addition of CFDA-labeled human apoptotic PMNs (3×105/well, 60 min, 37ºC). Increase in efferocytosis was determined by monitoring total fluorescence from PMNs; mean ± SEM, *p < 0.05 vs. vehicle, n = 3 healthy subjects.