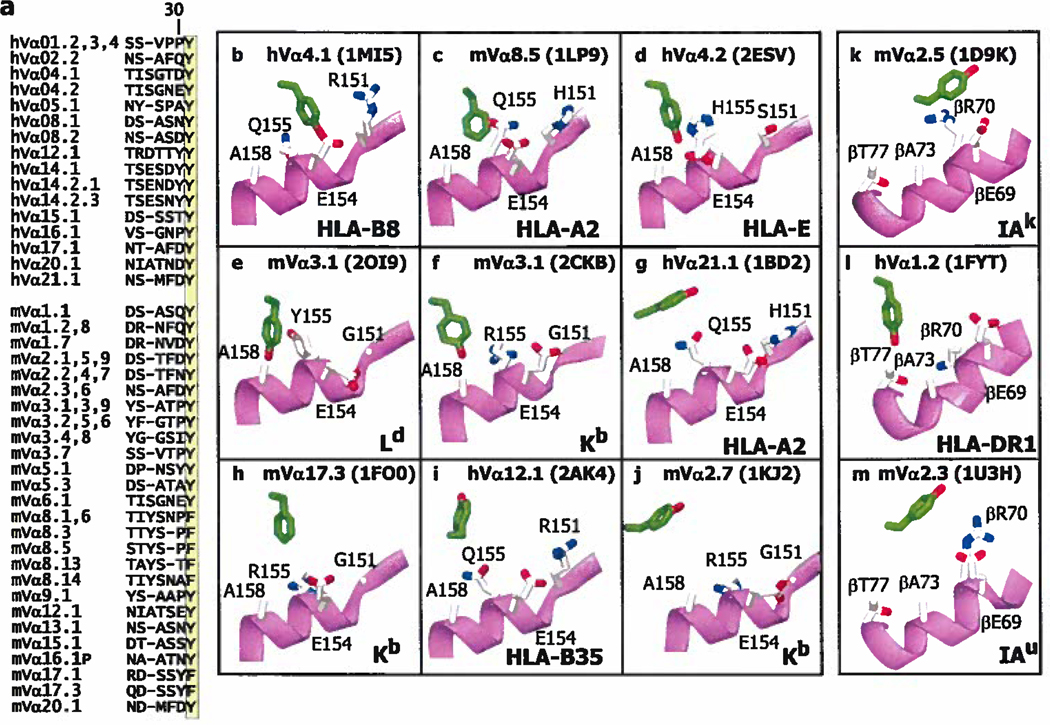
Figure 3. Y/F31 in CDR1α is often used in TCRs and often engages MHC at the same site.
a. The Figure lists the CDR1 sequences of the human (h) and mouse (m) Vα regions that contain an F or Y at position 31 (highlighted). V regions were omitted if the sequence of both their CDR1 and CDR2 regions were identical to one already displayed. The numbering of the V regions and their amino acids is according to (63, 64). The sequences were selected from those of 48 human Vαs and 75 mouse Vαs. b–m. Shown are the arrangements of MHC amino acids around Y/F31α (in green with red hydroxyl) in 9 (b–j) of the solved structures of TCRs bound to MHCI with the α2 helix of MHCI in magenta. Also indicated are the Vα region, MHC allele and pdb number. The structures are arranged from left to right and top to bottom roughly according the predicted strength of the interaction between Y/F31α and MHCI, with no predicted contact for Y/F31α in 2AK4 and 1KJ2, at the bottom of the Figure, k–m. Structures are shown as in b–j, but for TCRs bound to MHCII and with the β helix of MHCII in magenta. There is no predicted contact between Y/F31α and MHCII in 1U3H. Structures were selected from the references in Figure 2.