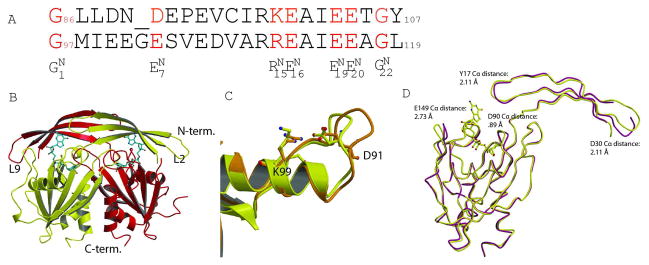
Fig. 1. Structure of GDPMK dimer.
A. Structural alignment of Nudix signature sequence of GDPMK (top) and EcADPRase (bottom). Signature sequence residues are colored red. B. Dimer of GDPMK with GDP mannose bound in the active site. C.The first loop of the Nudix motif is shorter in GDPMK than in EcADPRase. GDPMK is colored yellow, EcADPRase orange. D. Structural superposition of a monomer of apo form (D152A GDPMK) and the holo form (E100A GDPMK)of GDPMK.