Figure 1.
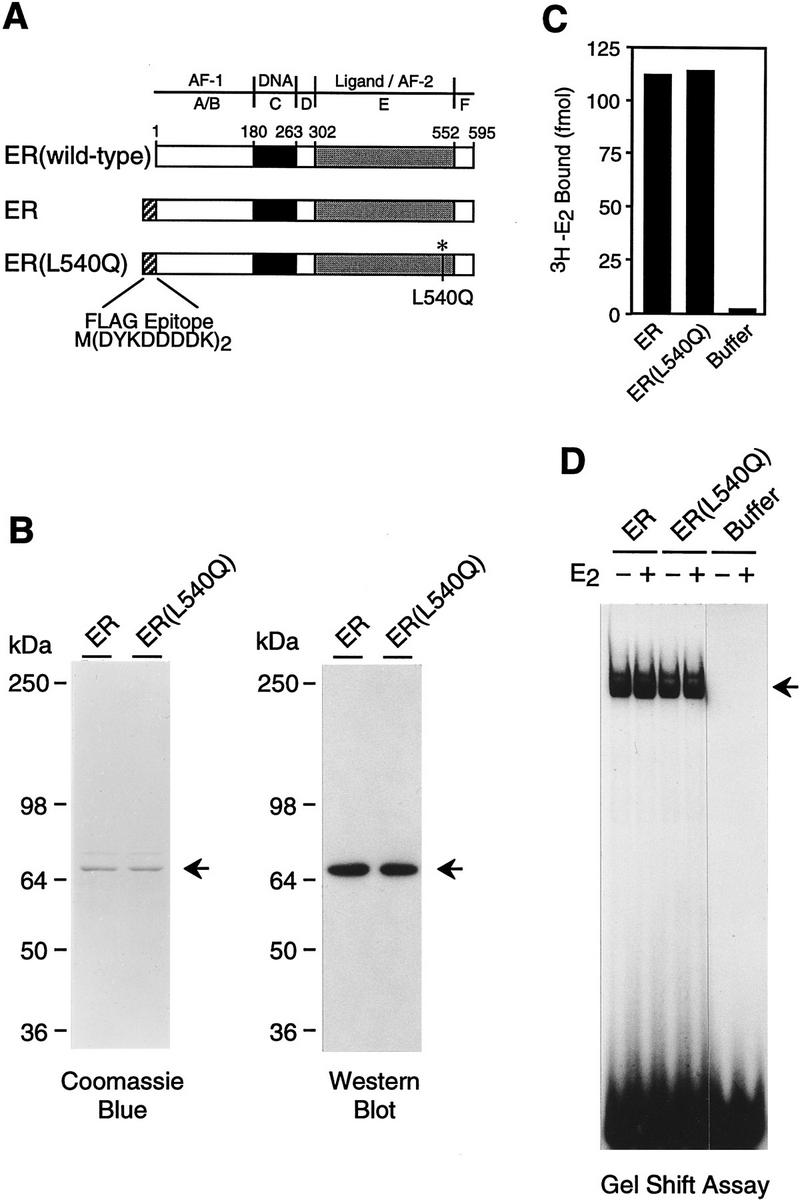
Purification of human ER and L540Q variant. (A) Schematic diagram of wild-type and epitope-tagged ERs. The locations of the amino-terminal Flag epitope tag and the Leu-540 → Gln (L540Q) point mutation are indicated. (B) Synthesis and purification of epitope-tagged ERs. Flag-tagged ER and ER(L540Q) were synthesized in Sf9 cells by using a baculovirus expression vector and affinity-purified with monoclonal antibodies that recognize the FLAG epitope. The proteins were subjected to 8% polyacrylamide–SDS gel electrophoresis. (Left) Total protein was visualized by staining with Coomassie Brilliant Blue R-250; (right) ERs were detected by Western blot analysis with anti-ER antibodies. (C) Ligand-binding activity of purified recombinant ERs. The 3H–E2 binding activity of approximately equimolar amounts of purified ERs was determined by ligand binding assays. Specific binding activity was determined by analyzing a second set of samples in the presence of a 200-fold excess of unlabeled E2. The net binding of 3H–E2 (in fmoles) is shown. (D) DNA-binding activity of purified recombinant ERs. Gel mobility shift assays were performed with approximately equimolar amounts of purified ERs. The samples were incubated with a 32P-end-labeled double-stranded oligonucleotide containing a consensus ERE sequence in the presence or the absence of E2 and then analyzed with a nondenaturing 4.8% polyacrylamide gel.
