Table 1.
Catalyst and Reaction Optimization
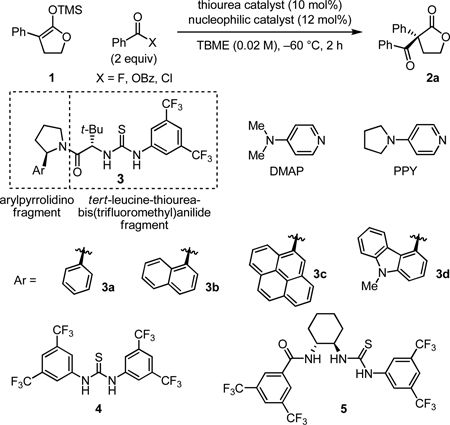 | |||||
|---|---|---|---|---|---|
| entrya | thiourea catalyst |
nucleophilic catalyst |
acylating agent (X =) |
yieldb (%) |
eec (%) |
| 1 | 3a | DMAP | OBz | 19 | 41 |
| 2 | 3a | PPY | OBz | 21 | 52 |
| 3 | 3a | PPY | Cl | 0 | - |
| 4 | 3a | PPY | F | 84 | 81 |
| 5 | 3b | PPY | F | 88 | 87 |
| 6 | 3c | PPY | F | 29 | 75 |
| 7 | 3d | PPY | F | 80 | 92 |
| 8 | 4 | PPY | F | 9 | - |
| 9 | 5 | PPY | F | 20 | <5 |
| 10d | 3d | PPY | F | 86 | 93 |
Reactions run on a 0.08 mmol scale.
Yields determined by 1H NMR analysis relative to p-xylene as an internal standard.
Enantiomeric excess determined by HPLC analysis on commercial chiral columns.
Reaction run using 5 mol% thiourea catalyst and 6 mol% PPY at −60 °C in TBME (0.01 M) for 8 h.
