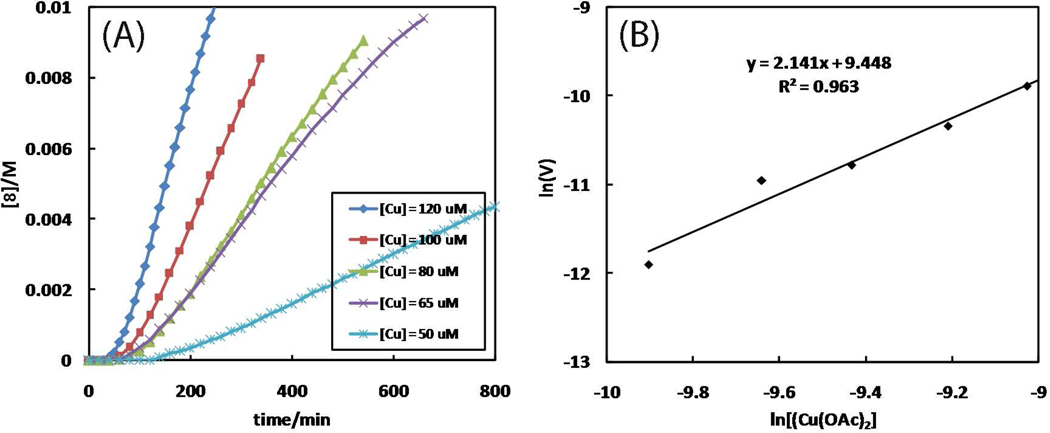
Figure 11.
(A) The dependence of reaction time course on [Cu(OAc)2·H2O]. Conditions: [1] = 25 mM, [Cu(OAc)2·H2O] = 0.05–0.12 mM, and [7] = 25 mM in CD3CN at 25 °C. Full conversions of each reaction would afford 8 at 25 mM. (B) Plot of ln Vint vs. ln[Cu(OAc)2·H2O]. The slope yields the kinetic order of Cu(OAc)2·H2O. Vint: initial observed rate = d[8]/dt (M·s−1).