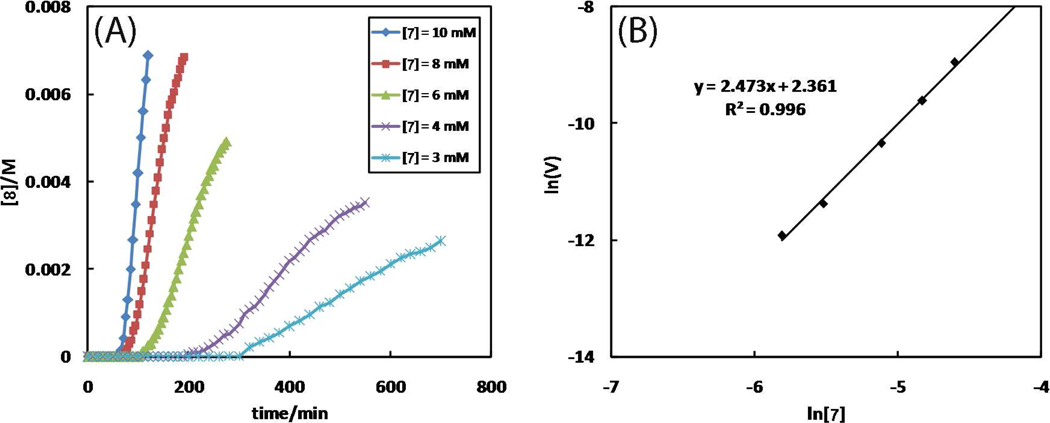
Figure 9.
(A) The dependence of reaction time course on [7]. Conditions: [1] = 10 mM, [Cu(OAc)2·H2O] = 1 mM, and [7] = 3–10 mM in CD3CN at 25 °C. Full conversions of each reaction would afford 8 at 3, 4, 6, 8, and 10 mM, respectively. (B) Plot of lnVint vs. ln[7]. The slope yields the kinetic order of 7. Vint: initial observed rate = d[8]/dt (M·s−1).