Table 2.
Effect of alkyne on the Cu(OAc)2-accelerated reactions involving chelating azides 1–3.a
| Entry | Timeb | Timeb | Timeb | |
|---|---|---|---|---|
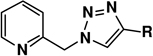 |
||||
| 1 | < 5 min | < 5 min | 8 h (84%)c | |
| 2 | < 5 min | 5–6 min | 2.5 h | |
| 3 | < 5 min | < 5 min | 8 h | |
| 4 | < 5 min | < 5 min | 30 min | |
| 5 | < 5 min | 8 h (93%)c | 8 h (75%)c | |
| 6 | 2.5 h | 25 min | 8 h (94%)c | |
| 7 | 8 h (84%)c | 8 h (96%)c | 2 h |
Reaction conditions: azide (0.2 mmol), alkyne (0.22 mmol), Cu(OAc)2 (5 mol%), in CH3OH (0.5 mL), rt. For reactions involving azide 3, TEA (0.2 mmol) was included.
Time for azide to disappear on TLC, followed by the confirmation of a full conversion (> 95%) by 1H NMR.
Incomplete conversion with percentage yield in parenthesis.
