Abstract
Background
In antiretroviral therapy (ART) programs, decreasing loss to follow up (LTFU) is a major priority.
Methods
We conducted a prospective study in Abidjan. Adults who started ART between June 2005 and May 2008 and were still in care 6 months later had monthly visits, biannual CD4 counts, computerized data collection and home visits (routine follow-up). A subset of patients also had biannual plasma HIV-1 RNA measurements, with a physician and a research assistant hired to pay particular attention to their visits (enhanced follow-up). We analysed the association between 18-month outcomes and pre-ART characteristics, medication possession ratio (MPR) and type of follow-up. Patients were LTFU if their last visit was before month-18 and they had not returned to care by month-24.
Results
2,074 patients started ART, of whom 1,636 (79%) were still in care at month-6. The routine (n=999) and enhanced (n=637) groups had similar baseline characteristics. From month-6 to month-18, they had similar death rates (routine 3.6%, enhanced 3.9%, p=0.74), but the enhanced group had significantly less LTFU (routine 11.3%, enhanced 5.8%, p<0.001). In multivariate analysis, the risk of LTFU from month-6 to month-18 was 46% lower with enhanced follow-up, 56% higher in patients living outside the centre area, and 4.0 fold higher in patients with a low MPR (<80%) between ART initiation and month-6.
Conclusion
In patients still in care at 6 months, a low MPR in the first 6 months was strongly associated with further LTFU. Simple follow-up enhancement halved the LTFU rate in the following year.
INTRODUCTION
Over the past six years, three million HIV-infected adults have started antiretroviral therapy in sub-Saharan Africa1. This unprecedented scaling up has been successful not only in saving lives but also in building capacity and allowing people to acquire experience in how to implement large treatment programs2.
Successful ART programs require providing lifelong treatment. A critical issue is how to help people maintain treatment over the long term. Reports from ART programs across sub-Saharan Africa have consistently shown that a substantial proportion of patients who initiate ART are then lost to follow-up (LTFU)3–5. Some of these patients died 6–10, while other are alive but have withdrawn from care for various reasons6, 11, 12. Preventing the latter from stopping treatment, or getting them back into care once they have stopped is an important priority for ART programs1.
Even if there is wide variation in reported rates of LTFU13, 14, some factors associated with LTFU have been consistently reported 6, 7, 11, 12, 15, 16. However, it is not easy to draw lessons, predict and identify which intervention should be implemented to decrease rates of LTFU in a specific setting 17.
The objective of this study was to assess whether simple enhanced follow-up procedures decreased LTFU rates in a large HIV care clinic in Côte d’Ivoire. A secondary objective was to examine factors associated with LTFU from month-6 to month-18 in this clinic, and determine whether some of them might help identify patients at month-6 for subsequent intervention(s) to prevent further LTFU.
METHODS
Patients
The CePReF clinic is the HIV care reference center of the Aconda program in Abidjan, Côte d’Ivoire 18,19. All patients who started ART at the CePReF clinic between June 6, 2005 and May 1, 2008 and attended their 6-month follow-up visit were eligible for the study.
Standard care and treatment
The standard of care for HIV-infected adults on ART in the Aconda program has been described elsewhere 19. During the study period, all patients initiated ART according to World Health Organization (WHO) criteria20. When patients were HIV-1-infected, first-line ART consisted of two nucleoside reverse transcriptase inhibitors (NRTI) and one non-nucleoside reverse transcriptase inhibitor (NNRTI). When they were HIV-2 or HIV-1 and HIV-2 dually reactive, first-line ART consisted of two NRTIs and one protease inhibitor (PI). CD4 counts and complete blood counts were measured every six months. Patients paid a fixed rate of US$2 per month for antiretroviral drugs and laboratory tests until August 2008, when the national HIV program made them available free of charge for patients. A team of six persons, including three social workers and 3 counselors, organized support groups to encourage patients to adhere to therapy 21and made telephone calls or home visits when patients did not show up to pick up their antiretroviral drugs 12, 22.
Computerized data recording system
Standardized forms were used to record the following variables at routine visits: (i) initial visit: date, sex, date of birth (or age), height, weight, HIV type (HIV-1, HIV-2, or both); follow-up visit: date, weight; (iii) ART initiation visit: date, WHO clinical stage, weight, patient’s home location; (iv) drug prescription (antiretroviral or other): date, name and quantity of drugs delivered; (v) CD4 count and complete blood count measurement: date, CD4 count, CD4 percentage, hemoglobin level, and platelet, granulocyte and leukocyte counts; (v) telephone call and home visit: dates at which patients were contacted, and vital status on that date; (vi) patients known to have died: date of death.
Enhanced follow-up
In June 2005, we launched a prospective cohort study (VOLTART cohort) of long-term virologic outcomes on ART in three HIV outpatient clinics in Abidjan, including the CePReF clinic 18. HIV-infected adults who started ART between June 6, 2005, and May 1, 2007 and attended their six-month visit were eligible for the cohort. Participants in VOLTART received the same standard care and treatment as other HIV-infected patients on ART. In addition, they received free plasma HIV-1 RNA every six months, a research physician was devoted to specifically attend the patients included in the cohort, and a research coordinator was devoted to monitoring and managing the cohort data and helping track patients by telephone and/or home visit.
Statistical analysis
In this study, we compare outcomes at month-18 in patients who participated in the VOLTART cohort (enhanced follow-up) and in those who attended their 6-month follow-up visit and received treatment at the CePReF clinic outside the framework of the cohort (routine follow-up). Outcomes were death, transfer of care, and LTFU.
Patients were defined as LTFU between month-6 and month-18 if: (i) their last contact with study team was before month 18; (ii) they were not known to be dead or transferred out between month-6 and month-18; (iii) no further information on their vital status could be obtained between month-18 and month-24, or they were found to be dead or transferred out between month-18 and month-24 and their date of death or date of transfer was more than 6 months after the date of their last contact with the study team.
The medication possession ratio (MPR) was defined as the number of daily doses of antiretroviral drugs dispensed by the pharmacy to each patient, divided by the patient’s total follow-up time in days since ART initiation.
Time to follow-up during the period of observation between routine and enhanced follow-up was compared by using time-to-first-event analyses, including Kaplan-Meier estimates and log-rank testing.
We used Cox proportional hazard regression analysis to estimate the association between the probability of being LTFU between month-6 and month-18 and sex, age, ART regimen, pre-ART CD4 count, pre-ART body mass index, distance living from the clinic, type of follow-up and the MPR between ART start and month-6 (M0-M6 MPR). Analyses were performed with SAS® software, version 9.1 (SAS institute Inc. Cary, North Caroline,USA).
RESULTS
Patients
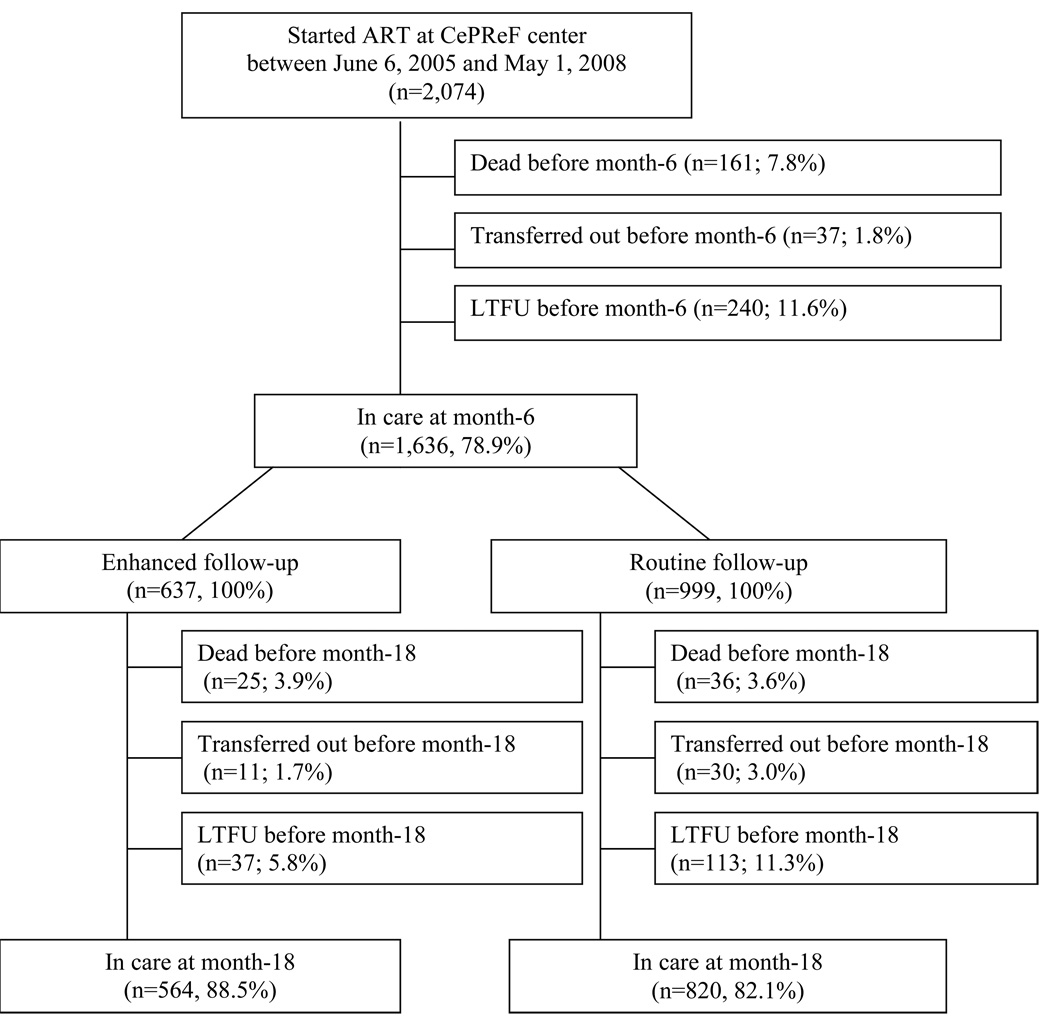
Overall, 2,074 adults started ART at the CePReF clinic during the study period. After 6 months on ART, 1,636 patients (78.9%) were still alive and in care. Of these, 637 were included in the VOLTART cohort (enhanced group) and 999 were followed under standard conditions (routine group) (Figure 1).
Figure 1. Flow chart of routine and enhanced follow-up in the Aconda ART program in Abidjan, Côte d’Ivoire.
Legend to Figure 1:
Lost to follow up before month 6: (i) last contact with study team <month-6; (ii) not dead or transferred out before month-6; (iii) no further information on vital status being obtained during the 6 following months (between month-6 and month-12), or dead or transferred out between month-6 and month-12 and date of death or date of transfer >6 months after date of last contact with study team.
Lost to follow up before month 18: (i) last contact with study team <month-18; (ii) not dead or transferred out between month-6 and month-18; (iii) no further information on vital status being obtained during the 6 following months (between month-18 and month-24), or dead or transferred out between month-18 and month-24 and date of death or date of transfer >6 months after date of last contact with study team.
ART: antiretroviral therapy; LTFU: lost to follow-up
Withdrawal from care before month-6
Patients who withdrew from care before month-6 had lower pre-ART CD4 count (p<0.001), lower BMI (p<0.001) and were more likely to be male (p<0.001) than patients still in care at month-6 (Table 1). Among patients who started ART during the study period, 7.8% died, 11.6% were lost to follow-up and 1.8% transferred care to another center before month-6. These outcomes did not change significantly over calendar time. The rate of death at month-6 was 8.5% in the February 2006–April 2007 period and 6.1% in the May 2007–May 2008 period (p=0.06). The rate of LTFU at month-6 was 11.1% in the February 2006–April 2007 period and 12.6% in the May 2007–May 2008 period (p=0.34).
Table 1.
Characteristics of patients starting ART at the CePReF HIV care center, Abidjan, Côte d’Ivoire, 2005–2008
| Dead, transferred out or LTFU before Month-6 (n=438) |
Still in care at Month-6 |
|||
|---|---|---|---|---|
| Enhanced follow-up (n= 637) |
Routine follow-up (n=999) |
p-value | ||
| Female, n (%) | 289 (66) | 475 (75) | 741 (74) | 0.88 |
| Age in years, median (IQR) | 36 (30–43) | 37 (31–43) | 36 (30–43) | 0.50 |
| Living outside the district, n (%) | 153 (35) | 215 (34) | 360 (36) | 0.32 |
| Pre-ART Body Mass Index (kg/m2), median (IQR) | 18.0 (16.0–20.2) | 19.8 (17.8–22.1) | 20.1 (17.9–22.5) | 0.25 |
| Pre-ART CD4 count (/mm3), median (IQR) | 97 (25–194) | 148 (71–226) | 147 (58–233 ) | 0.69 |
| First-line ART regimen, n (%) | 0.04 | |||
| 2 NRTIs + 1 NNRTI | 417 (95) | 619 (97) | 952 (95) | |
| 2 NRTIs + 1 PI | 19 (4) | 16 (3) | 42 (4) | |
| 3 NRTIs | 1 (0) | 0 (0) | 5 (1) | |
| M0-M6 Medication Possession Ratio, median (IQR) | 74 (33–100) | 95 (83–99) | 96 (81–101) | 0.09 |
ART: antiretroviral therapy; IQR : inter-quartile range ; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: Protease inhibitor. LTFU: lost to follow-up.
p-value is for comparison between routine and enhanced follow-up between 6 and 18 months among patients still in care at month-6.
M0-M6 Medication Possession Ratio: number of daily doses of antiretroviraldrug provided between ART start and month-6, divided by total number of follow-up days between ART start and month-6
Outcomes from month-6 to month-18
Pre-ART characteristics and M0-M6 MPR were similar in patients from the enhanced and routine groups (Table 1).
Between month-6 and month-18, patients in the enhanced and routine groups had similar rates of death (3.9% vs. 3.6%, p=0.74) and transfer out (1.7% vs. 3.0%, p=0.11), while patients from the enhanced group were less likely to be LTFU than those in the routine group (5.8% vs. 11.3%, p<0.001) (Figure 1).
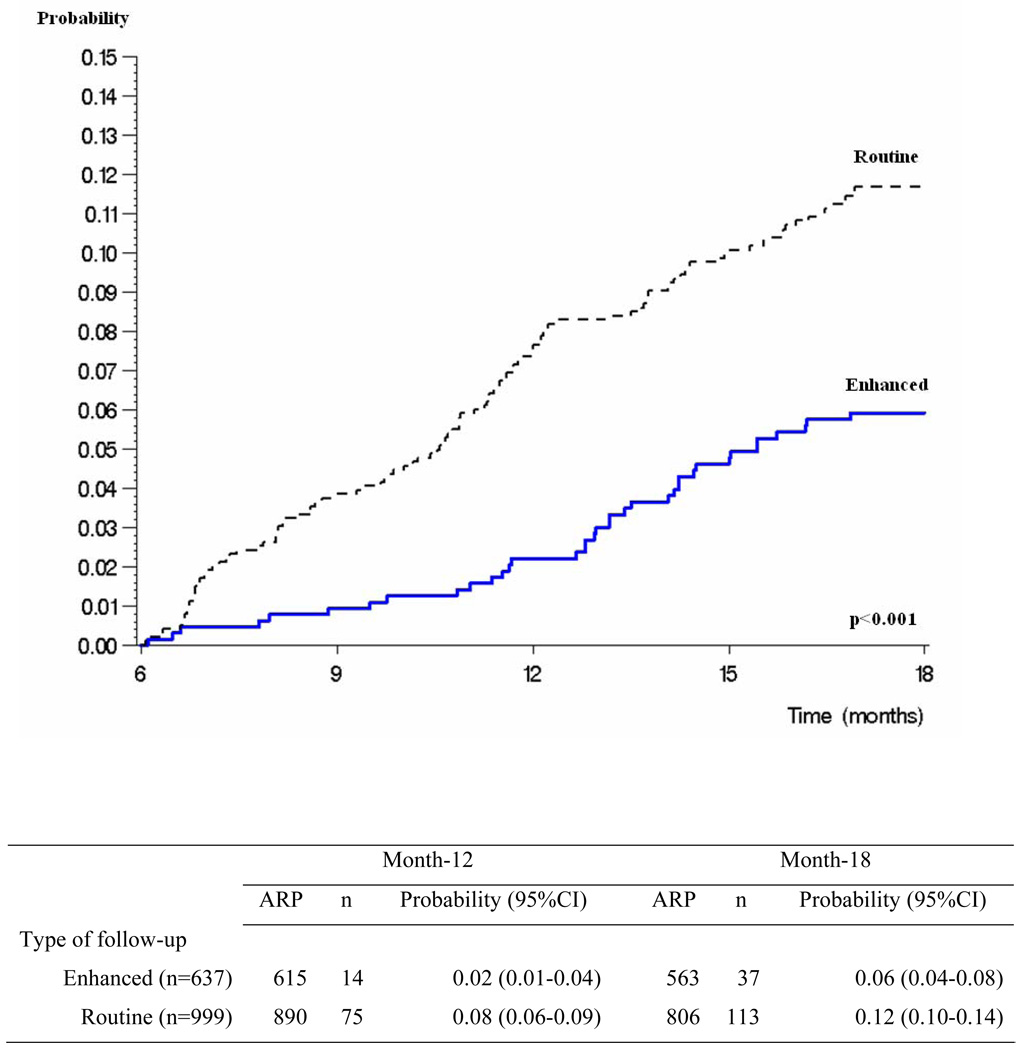
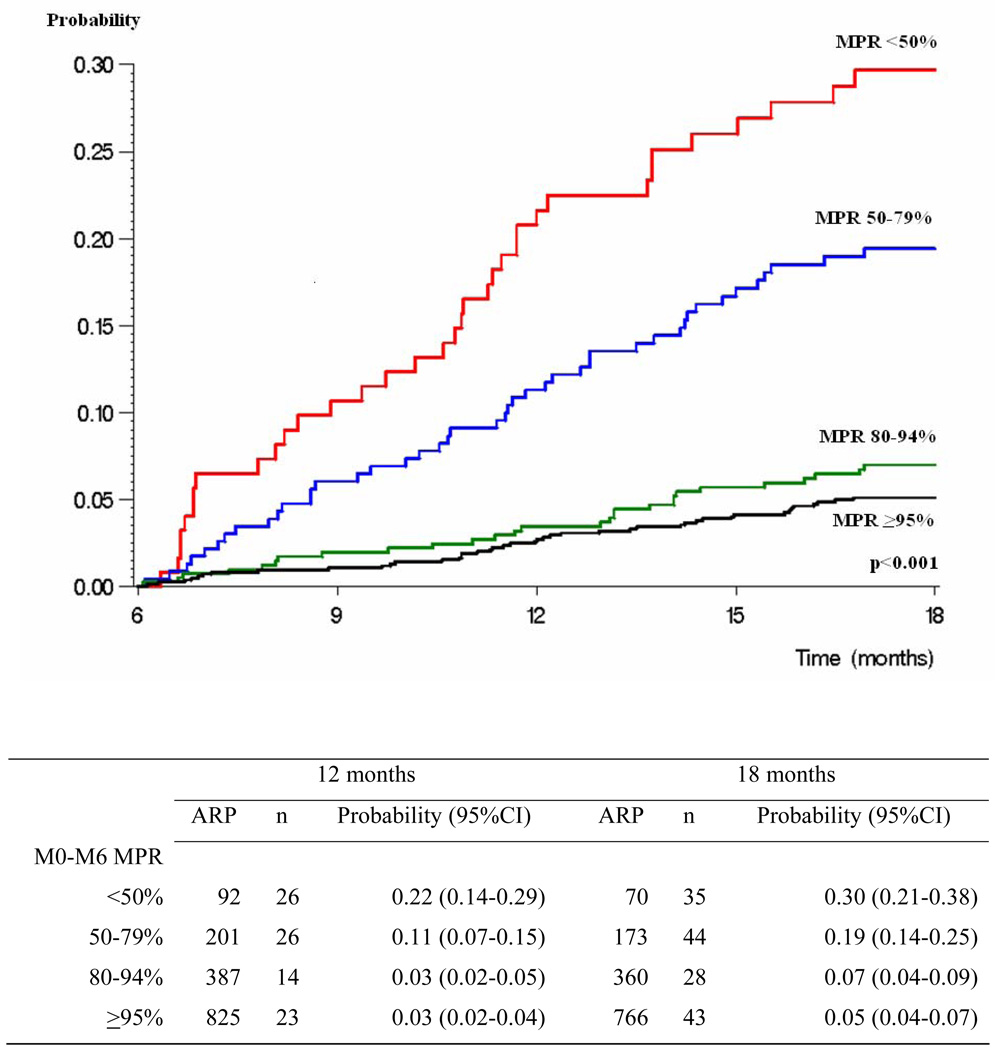
In multivariate analysis, the probability of LTFU was lower in the enhanced group, in patients living within the boundaries of the district centered on the clinic, and in those with higher M0-M6 MPR (Table 2). Patients in the enhanced group had a 46% decrease in the risk of being LTFU from month-6 to month-18 (HR: 0.54; CI: 0.37–0.78), those living outside the study center area had a 53% increase in the risk of being LTFU from month-6 to month-18 (HR: 1.53; CI: 1.10–2.13), and those having a M0-M6 MPR at 80–94%, 50–79% and <50% had a 1.5, 4.0 and 5.7 fold increase in the risk of being LTFU from month-6 to month-18 compared to those having a M0-M6 MPR ≥95%. The probability of being LTFU between month-6 and month-18 was 12% in the routine and 6% in the enhanced groups (Figure 2). The probability of being LTFU between month-6 and month-18 was 5%, 7%, 19% and 30% in patients with M0-M6 MPR ≥95% 80–94%, 50–79% and <50% (Figure 3).
Table 2.
Factors associated with the risk of being lost to follow up between Month-6 and Month-18 in patients in care at Month-6, CePReF HIV care center, Abidjan, Côte d’Ivoire
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | (95% CI) |
p- value |
AHR | (95% CI) |
p- value |
|
| Sex (men vs. women) | 1.12 | (0.78–1.60) | 0.54 | - | - | - |
| Age in years (≤35 vs. ≥35) | 1.39 | (1.01–1.93) | 0.04 | 1.29 | (0.93–1.81) | 0.13 |
| Living outside the district (yes vs. no) | 1.44 | (1.04–1.99) | 0.03 | 1.53 | (1.10–2.13) | 0.01 |
| Pre-ART body mass index (<19.5 vs. ≥ 19.5 kg/m2) | 1.08 | (0.78–1.49) | 0.64 | - | - | - |
| Pre-ART CD4 count | 0.06 | 0.06 | ||||
| < 100 vs. ≥ 200/mm3 | 0.95 | (0.63–1.44) | 0.82 | 1.02 | (0.67–1.55) | 0.91 |
| 100–199 vs. ≥ 200/mm3 | 1.46 | (0.99–2.16) | 0.06 | 1.53 | (1.03–2.27) | 0.04 |
| First ART regimen (PI-based vs. others) | 1.55 | (0.76–3.17) | 0.22 | - | - | |
| M0-M6 Medication Possession Ratio | 0.0001 | 0.0001 | ||||
| <50 vs. ≥95% | 6.96 | (4.45–10.88) | 0.0001 | 5.74 | (3.60–9.15) | 0.0001 |
| 50–79 vs. ≥95% | 4.16 | (2.73–6.33) | 0.0001 | 4.02 | (2.62–6.16) | 0.0001 |
| 80–94 vs. ≥95% | 1.38 | (0.86–2.22) | 0.18 | 1.46 | (0.90–2.35) | 0.13 |
| Type of follow-up (enhanced vs. routine) | 0.48 | (0.33–0.70) | 0.0001 | 0.54 | (0.37–0.78) | 0.001 |
HR: Hazard Ratio; AHR: adjusted Hazard Ratio; CI: Confidence interval; PI: Protease inhibitor
M0-M6 Medication Possession Ratio: number of daily doses of antiretroviraldrug provided divided by total number of follow-up days since ART initiation
Figure 2. Probability of being lost to follow up between Month-6 and Month-18 in patients in care at Month-6, by type of follow-up.
Legend to Figure 2:
ARP: number of at risk patients
n: number of events
CI: confidence interval
Figure 3. Probability of being lost to follow up between Month-6 and Month-18 in patients in care at Month-6, by M0-M6 medication possession ratio.
Legend to Figure 3:
ARP: number of at risk patients; n: number of events; CI: confidence interval
M0-M6 MPR: number of daily doses of antiretroviral drug actually provided between ART start and month-6 divided by total number of follow-up days between ART start and month-6.
We did not find a significant association between LTFU and sex, age, or pre-ART CD4 counts.
DISCUSSION
We conducted a prospective cohort study nested in a large HIV care programs in Abidjan, Côte d’Ivoire. In this program, during the first year of the study period, adult patients who had received ART for 6 months and remained in care were offered participation in the cohort study on the day of their 6-month visit. Cohort participants received the same care and treatment as non-participants, and had the same follow-up routine monitoring procedures, which included monthly visits, CD4 counts every 6 months and home visits or phone calls when they did not show up to pick up their drugs. However, from 6 months onward, follow-up of participants was enhanced in two ways: first, participants had plasma HIV-1 RNA measured at six months, and then every six months thereafter; second, a dedicated team of two persons enhanced their follow-up. A physician was devoted to attend to patients included in the cohort whenever they showed up for their monthly visits; and a research assistant was devoted to following the cohort participants’ data and ensuring that those who missed a pharmacy refill were tracked by telephone and/or home visit. Of note, participants in the cohort, as well as non-participants, had to pay for their transportation, for all care and treatment other than ARV drugs and for all tests other than those included in routine monitoring.
We compared 18-month outcomes of participants and non participants in the cohort, and had two main findings.
First, reinforcing follow-up beginning at month-6 was independently associated with a 47% decrease in the risk of LTFU between month-6 and month-18. It has been shown previously that patients participating in research studies have better outcomes compared to those treated in routine programs, including lower mortality and lower rate of LTFU. However, this cohort was not a typical research study, in the sense that participants had the same standard of care as non-participants. In what is usually known as the “cohort effect”, free transportation, free care, including drugs and tests, more frequent scheduled visits, and specific cohort-associated procedures to track LTFU, are often thought to explain why cohort participants have better outcomes than patients followed in routine programs 9. In this study, cohort participants had none of these advantages compared to non-participants. There are three potential reasons why they were less likely to be LTFU. First, measuring viral load every six months could decrease the rate of LTFU in the year following the first measurement. If this is true, one would expect viral load measurement to also decrease mortality, which we did not observe. Second, being consistently attended to by the same physician could encourage patients to remain in care. Third, a dedicated research assistant for each 600 patients could optimize tracking procedures, even in a care center where a team of 6 persons is already routinely in charge of making sure these procedures are applied.
The second key finding of the study is that the MPR during the first six months of ART was strongly associated with the risk of LTFU from month-6 to month-18 in patients who were in care at month-6, in both the routine and enhanced groups also show elsewhere 23. In our center, procedures to reinforce adherence at 6 months in patients who are believed to be non- adherent are already in place. Some of the study patients who had low medication possession ratio probably benefited from these procedures. However, at the time of the study, the medication possession ratio was not explicitly identified as a tool to predict the short-term risk of being LTFU. This strongly suggests that the MPR at month-6 can be used to identify those at highest risk of LTFU and develop specific interventions to improve their retention24, 25.
This study has several limitations. First, patients were not randomly assigned to routine or enhanced follow-up; these were offered to them on a calendar time basis. Therefore, we cannot rule out changes over time in the way patients were followed in this HIV care center2. However, the outcomes during the first six months of ART did not change over calendar time during the study period, and neither did the 6–18 month rates of death and of patient transfer. It is unlikely, therefore, that a change in study center characteristics over that time explains the 47% decrease in the rate of LTFU. Second, the number of variables included in the analysis was limited. Some variables not recorded might have acted as confounders in the analysis, even if patients in the routine and enhanced groups were similar in terms of measured characteristics. Because we adjusted the analysis for age, sex, ART regimen, pre-ART CD4 count, home location, and MPR during the first six months, we believe that major confounding was controlled for.
In HIV-infected adults alive and in care after 6 months of ART, the MPR can be used routinely at month-6 to identify patients who may benefit most from follow-up reinforcement. Furthermore, additional simple follow-up enhancement procedures halved the risk of LTFU from month-6 to month-18.
Acknowledgments
Funding: Agence Nationale de Recherches sur le SIDA et les hepatites virales (ANRS 12136, ANRS 12212) and National Institute of Allergy and Infectious Diseases (R01 AI058736, K24 AI062476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. Available from http://wwwwhoint/hiv/pub/2010progressreport/report/en/indexhtml.
- 2.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 3.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010;88:593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekouevi DK, Balestre E, Ba-Gomis FO, Eholie SP, Maiga M, Amani-Bosse C, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15 Suppl 1:34–42. doi: 10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are "lost to follow-up" in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 8.Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglaret X, Toure S, Gourvellec G, Tchehy A, Zio L, Zaho M, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 10.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15 Suppl 1:63–69. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–857. [PubMed] [Google Scholar]
- 12.Weigel R, Hochgesang M, Brinkhof M, Hosseinipour M, Boxshall M, Mhango E, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen S, Fox MP, Gill CJ. Patient Retention in Antiretroviral Therapy Programs in Sub-Saharan Africa: A Systematic Review. PLoS Med. 2007;4:1691–1701. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 Suppl 1:1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12:687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 16.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88:681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losina E, Toure H, Uhler LM, Anglaret X, Paltiel AD, Balestre E, et al. Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: a Cote d'Ivoire appraisal. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000173. e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messou E, Chaix M, Gabillard D, Minga A, Losina E, Yapo V, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1 infected adults on antiretroviral therapy in Côte d'Ivoire. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3182084b5a. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messou E, Anglaret X, Duvignac J, Konan-N'dri E, Komena E, Gnokoro J, et al. Antiretroviral treatment changes in adults from Cote d'Ivoire: the roles of tuberculosis and pregnancy. AIDS. 2010;24:93–99. doi: 10.1097/QAD.0b013e32832ec1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006 revision. Available from http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf. [PubMed]
- 21.Decroo T, Telfer B, Biot M, Maïkéré J, Dezembro S, Cumba L, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete province, Mozambique. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3182055138. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–122. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 24.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon J, Jordan M, Kelley K, Bertagnolio S, Hong S, Wanke C, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011;52:493–506. doi: 10.1093/cid/ciq167. [DOI] [PMC free article] [PubMed] [Google Scholar]