Table 3.
Ruthenium catalyzed transfer hydrogenative coupling of alkynes 6a–6c to aldehyde 4b (top) and alcohol 1b (bottom).a
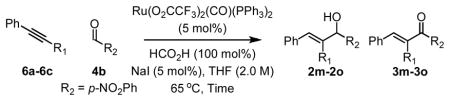 | ||||
|---|---|---|---|---|
| Entry | Alkyne (200 mol%) | Product | Time (hr) | Yield (2:3) |
| 1 | 6a, R1 = Ph | 2m (3m) | 24 hr | 91% (>20:1) |
| 2 | 6b, R1 = (CH2)2OBn | 2n (3n) | 16 hr | 84% (>20:1) |
| 3 | 6c, R1 = CH2NHBoc | 2o (3o) | 13 hr | 75% (>20:1) |
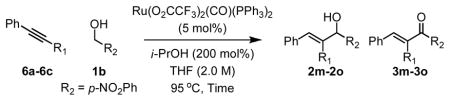 | ||||
|---|---|---|---|---|
| Entry | Alkyne (200 mol%) | Product | Time (hr) | Yield 2 (3) |
| 1 | 6a, R1 = Ph | 2m (3m) | 37 hr | 62% (12%) |
| 2 | 6b, R1 = (CH2)2OBn | 2n (3n) | 13 hr | 58% (>1%) |
| 3 | 6c, R1 = CH2NHBoc | 2o (3o) | 13 hr | 15% (>1%) |
See supporting information for detailed procedures. Isolated yields refer to pure 2m–2o free of any enone byproduct.
