Table 1.
Optimizing relative and absolute stereocontrol in transfer hydrogenative carbonyl crotylation from the alcohol oxidation level.a
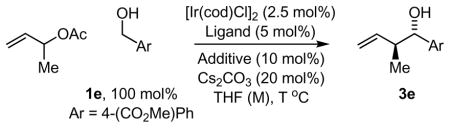 | |||||||
|---|---|---|---|---|---|---|---|
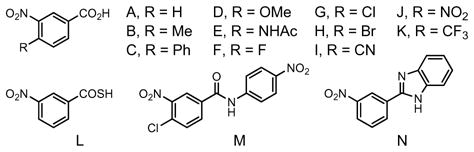
| Entry | Ligand | Acid | OAc (eq) | THF (M) | T °C | Y (%) | dr (ee%) |
| 1 | BIPHEP | A | 10 | 0.2 | 100 | 85 | 2.0:1 |
| 2 | BIPHEP | B | 10 | 0.2 | 100 | 72 | 2.7:1 |
| 3 | BIPHEP | C | 10 | 0.2 | 100 | 10 | 2.0:1 |
| 4 | BIPHEP | D | 10 | 0.2 | 100 | 68 | 2.2:1 |
| 5 | BIPHEP | E | 10 | 0.2 | 100 | 50 | 1.5:1 |
| 6 | BIPHEP | F | 10 | 0.2 | 100 | 78 | 2.3:1 |
| 7 | BIPHEP | G | 10 | 0.2 | 100 | 93 | 2.6:1 |
| 8 | BIPHEP | H | 10 | 0.2 | 100 | 80 | 2.4:1 |
| 9 | BIPHEP | I | 10 | 0.2 | 100 | 70 | 3.0:1 |
| 10 | BIPHEP | J | 10 | 0.2 | 100 | 65 | 3.5:1 |
| 11 | BIPHEP | K | 10 | 0.2 | 100 | 86 | 2.4:1 |
| 12 | BIPHEP | L | 10 | 0.2 | 100 | 38 | 1.9:1 |
| 13 | BIPHEP | M | 10 | 0.2 | 100 | 7 | 1.9:1 |
| 14 | BIPHEP | N | 10 | 0.2 | 100 | 5 | 2.1:1 |
| 15 | BIPHEP | I | 5 | 0.2 | 100 | 57 | 3.7:1 |
| 16 | BIPHEP | I | 2 | 0.2 | 100 | 55 | 4.3:1 |
| 17 | BIPHEP | I | 2 | 0.5 | 100 | 77 | 4.8:1 |
| 18 | BIPHEP | I | 2 | 1.0 | 100 | 75 | 7.1:1 |
| 19 | BIPHEP | I | 2 | 1.0 | 90 | 78 | 7.5:1 |
| 20 | BIPHEP | J | 2 | 1.0 | 90 | 42 | 7.6:1 |
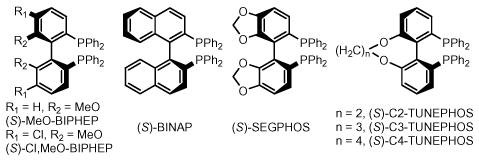
| 21 | (S)-BINAP | I | 2 | 1.0 | 90 | 75 | 3.5:1 (95) |
| 22 | (S)-MeO-BIPHEP | I | 2 | 1.0 | 90 | 63 | 5.8:1 (94) |
| 23 | S)-Cl,MeO-BIPHEP | I | 2 | 1.0 | 90 | 67 | 3.0:1 (96) |
| ⇨ 24 | (S)-SEGPHOS | I | 2 | 1.0 | 90 | 70 | 7.4:1 (95) |
| 25 | (S)-C2-TUNEPHOS | I | 2 | 1.0 | 90 | 68 | 7.7:1 (91) |
| ⇨ 26 | (S)-C3-TUNEPHOS | I | 2 | 1.0 | 90 | 77 | 8.0:1 (97) |
| 27 | (S)-C4-TUNEPHOS | I | 2 | 1.0 | 90 | 71 | 6.4:1 (92) |
|
| |||||||
| |||||||
| |||||||
All reactions were performed in 13 × 100 mm pressure tubes. The cited yields are of material isolated by silica gel chromatography. Enantiomeric excess was determined by chiral stationary phase HPLC analysis via comparison to racemic diastereomeric mixtures. For entries 1–18, the reaction was allowed to run for 20 hours. For entries 19–27, the reaction was allowed to run for 48 hours. See experimental section for further details.
