Abstract
The neonatal hypoxia-ischemia rat model referred to as the Rice–Vannucci model is extensively used to study perinatal hypoxia-ischemia and child brain injury. One of the major weaknesses of this model is its inconsistency of brain infarction among animals. We hypothesize that the inconsistency of infarction is caused by prolonged operation time and therefore isoflurane exposure. Neonatal hypoxia-ischemia was induced in postnatal days 7 and 10 rat pups by unilateral right common carotid ligation followed by 2.5 h of hypoxia (8% oxygen). The incision-to-ligation (ITL) was defined as the amount of time from initial incision (4 min after 2% isoflurane exposure) to completion of carotid ligation (at which point isoflurane exposure was also terminated). In the first part of the study, the ITL of each group was designated to be 5, 13, and 21 min. In the second part of the study, the ITL is designated to 4 min; however, continued isoflurane was used to make 5, 13, and 21 min isoflurane exposure for each group. Percentages of brain infarction were assessed at 48 h following surgery. Motor deficits were accessed by Rotarod test. Marked brain infarction was observed in the 5-min ITL group and a decrease of brain infarction observed in the 13-and 21-min groups (P<0.05). In the second part of the study, marked brain infarction was observed in the 5-min isoflurane exposure group, and a decrease of brain infarction was observed in each of the 13- and 21-min isoflurane exposure groups (P<0.05). Similar tendencies were observed in Rotarod tests than 5-min ITL and 5-min isoflurane groups showed more marked deficits (P<0.05). This study demonstrated that brain infarction inconsistency of the neonatal hypoxia-ischemia rat pup model is related to the operation time. The observed time-dependent decrease of brain infarction is correlated to the isoflurane exposure time. Shorter operation and isoflurane exposure improves this model consistency of brain infarction and motor deficits.
Keywords: Neonatal hypoxia-ischemia, Isoflurane, Neuroprotection, Rotarod test
Introduction
In human neonates, hypoxic-ischemic (HI) events cause brain injury which can lead to major neurologic deficits and impairment in growth and development [2]. To study neonatal HI, animal models have been developed to enable more intensive evaluation of neuroprotective agents. One easy and widely used neonatal HI rat model is unilateral common carotid artery ligation followed by exposure of the rat pups to hypoxia (about 8% oxygen). This is referred to as the Rice–Vannucci model [26]. While the surgery for Rice–Vannucci model is easy to perform, the major challenge met in adopting this model is developing a consistent volume of infarction across experimental subjects, in each of the most commonly used age groups of 7 and 10 days old [11, 14, 26, 31]. The younger group of animals, at 7 days of age, is to reflect the neonatal rat; while the 10-day-old rat is to model the older neonate or infant approaching adolescent stages of development. Consistency is key to utilizing this model for the study of different novel neuroprotective agents at varying time points and dosages [3–5, 9, 15–17, 33–36, 39–43].
The most commonly used anesthetic for the common carotid ligation procedure in rat pups is isoflurane, due to its safety, possible enhancement of perioperative survival [2], and convenience of handling for smaller animals such as 7- or 10-day-old rats. Isoflurane, along with other inhalational volatile anesthetics, has been demonstrated to possess neuroprotective properties against ischemic tissue injury [19, 35, 39–43], enhanced tolerance against ischemic injury [17, 21, 24, 25], reduced hypoxia-ischemia induced mortality and functional impairment [20, 23, 32], via involvement in different mechanisms associated with ischemic brain injury [8, 16, 34–36, 39–43], as well as potential reduction of brain edema [22]. This neuroprotective property of isoflurane has been demonstrated to be dose-dependent [34] which is of consideration when examining increased exposure times (such as in this model). There has also been evidence of protection against the effects of hypoxic-ischemic brain injury in the long term [41]. We hypothesize that the observed inconsistencies in infarction volumes in the Rice–Vannucci model are due to prolonged procedural times and the resulting increased duration of isoflurane exposure.
Materials and Methods
This protocol was evaluated and approved by the Institutional Animal Care and Use Committee at Loma Linda University, Loma Linda, California. Twelve Sprague–Dawley dams with litters (Harlan Laboratories) were received and maintained with their dams immediately prior to experimentation dates of 7 and 10 days postnatal age.
Surgical Procedure
Permanent unilateral common carotid ligation by the Levine unilateral procedure was performed on 216 rat pups. Following induction with 2% isoflurane anesthesia at 0.7 L/min for 4 min, aseptic preparation proceeded, and a longitudinal midline incision was made in the neck. The right common carotid artery was double ligated using 7-0 surgical silk and cut between the ligatures. Animals experiencing surgical complications including carotid laceration or other surgical site hemorrhage were excluded from the study. After completion of surgery, subjects recovered in a heated cage controlled to 38°C during a 1-h wait period to recover from anesthesia prior to placement in the hypoxic chamber.
Experimental Parameters
The incision-to-ligation (ITL) time was defined as the amount of time from initial skin incision to completion of carotid ligation (termination of isoflurane exposure). Following completion of the surgery, the pups were placed in a chamber where they were exposed to 2.5 h of hypoxia (8% oxygen). For the first 1.25 h, the 8% oxygen supply rate was 3.5 L/min and for the remaining 1.25 h the rate was 4.0 L/min. These parameters were used to achieve significant and consistent infarction with longer exposures to hypoxia leading to greater extent of brain injury [32]. Vannucci et al. described the tolerance of the rat pups to hypoxia for up to 3 h prior to appreciable mortality. Subjects that died during hypoxia comprised 12.5% (27 of 216) and did not continue in the study. The distribution of mortality was nearly equal across experimental groups and increased mortality was not observed in any of the experimental groups compared with others with statistical significance. There was no mortality after completion of the hypoxic chamber exposure. Perioperative warming of each animal was maintained consistently at 38°C to control for possible neuroprotection by hypothermia and ambient temperature fluctuations [3, 28, 32, 37].
Experimental Groups
In part one of the study, the ITL time randomly assigned to each subject was 5, 13, or 21 min. All animals underwent 4 min of induction time in isoflurane. The surgery then proceeded under continued isoflurane. Completion of ligation was intentionally delayed until the specified ITL time was reached. The carotid was exposed and isolated; then the suture was placed but not tied until 5, 13, or 21 min had elapsed from the initial incision. After ligation of the carotid, isoflurane was stopped, the incision site was closed, and the animal was allowed to recover. The total exposure time to isoflurane of each animal was therefore the sum of 4 min of induction plus the ITL time. Subjects were either examined for infarction at 48 h or evaluated for neurobehavior 28 days later.
In the second part of the study, the ITL time was set to 4 min. All animals underwent 4 min of isoflurane induction. Under continued isoflurane, surgery proceeded and ligation was completed at exactly 4 min for all subjects. After completion of the ligation, the subject continued to receive isoflurane exposure for an additional 1, 9, or 17 min. Surgical exposure to isoflurane therefore totaled 5, 13, or 21 min. The sum total of isoflurane exposure in this part of the study was the 4 min of induction plus 5, 13, or 21 min. Subjects were either examined for infarction at 48 h or evaluated for neurobehavior at 28 days afterwards. Matching groups of 7- and 10-day-old pups were used in this study to evaluate for potential differences between the effects of isoflurane on each of the two most commonly used age groups in this model as previously discussed.
The key difference between the two experimental parts is the duration of the surgery defined as the ITL. In part one, the surgical procedure itself lasted 5, 13, or 21 min. In part two, the surgical procedure lasted 4 min.
Evaluation of Infarction Volume
For infarction volume assessments, six groups of nine animals each were sacrificed at 48 h after completion of hypoxia. Whole brain specimens were immediately placed into the rat brain slicing matrix and sectioned into 2 mm thick coronal slices, which were incubated in triphenyltetrazolium chloride at 37°C for enhanced evaluation of ischemic infarction volumes. Digital imaging of the slices was then analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA; www.NIH.gov) for summation and calculation of infarction volumes.
Neurobehavioral Evaluation
Long-term behavioral testing was performed on six groups of nine animals. At 28 days after surgery and hypoxia, rotarod testing of motor coordination was done. As previously described [5], the rats were placed on an accelerating horizontal cylinder and the latency and rotational speed of each subject’s falling off was recorded by a blinded evaluator. Three constant velocity runs of 1 min each were done per subject prior to testing. Three scoring trials were performed at each of 5 or 10 rpm starting speeds with regular increases in velocity, with the mean averages of falling latency used for comparison. This was done to compare the degree of functional impairment resulting from brain injury [18].
Statistical Analysis
Statistical analyses were performed by one-way repeated measures of analysis of variance on ranks complemented by the Dunn method. P value ≤0.05 was accepted as significant for all analyses. Statistical analyses were performed using the SigmaStat software program (SYSTAT Software, Inc., Point Richmond, CA, USA).
Results
Part I
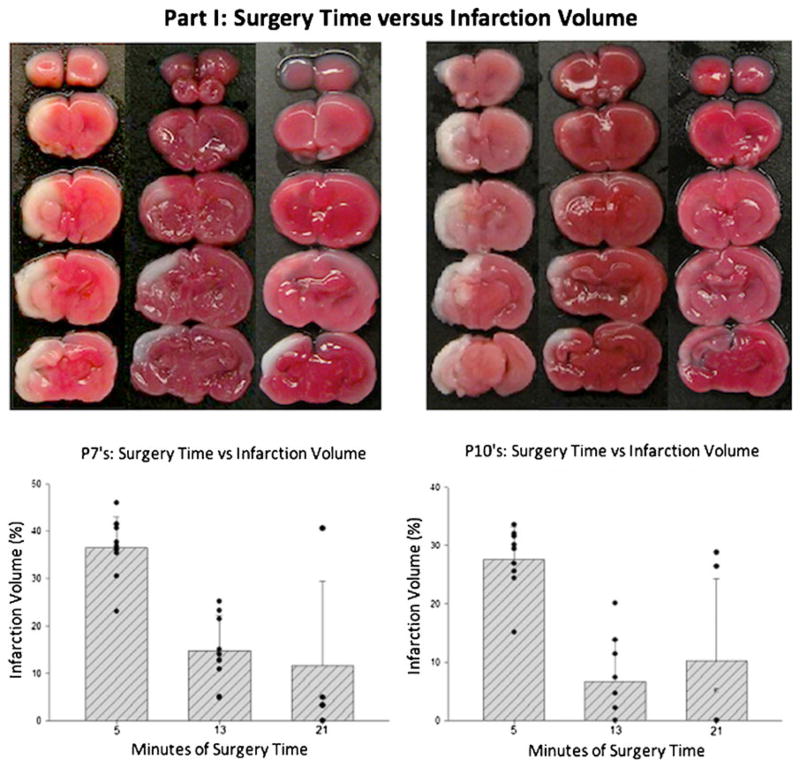
Surgery time versus infarction volume: In the first part of the study (Fig. 1), surgery time (ITL) was defined as the time point at which carotid ligation was made (5, 13, or 21 min). In the 7-day-old rat pups, the largest brain infarction volume (mean 36.4%, SD 0.066) was observed in the 5-min ITL group and significantly smaller volumes of brain infarction were observed in the 13-min (mean 14.7%, SD 0.074) and 21-min (mean 11.5%, SD 0.18) groups (P<0.05). For the post-natal day 10 pups (Fig. 1), the largest brain infarction volume (mean 27.6%, SD 0.056) was observed in the 5-min ITL group and significantly smaller volumes of brain infarction were observed in the 13-min (mean 6.6%, SD 0.07) and 21-min (mean 10.2%, SD 0.141) groups (P<0.05).
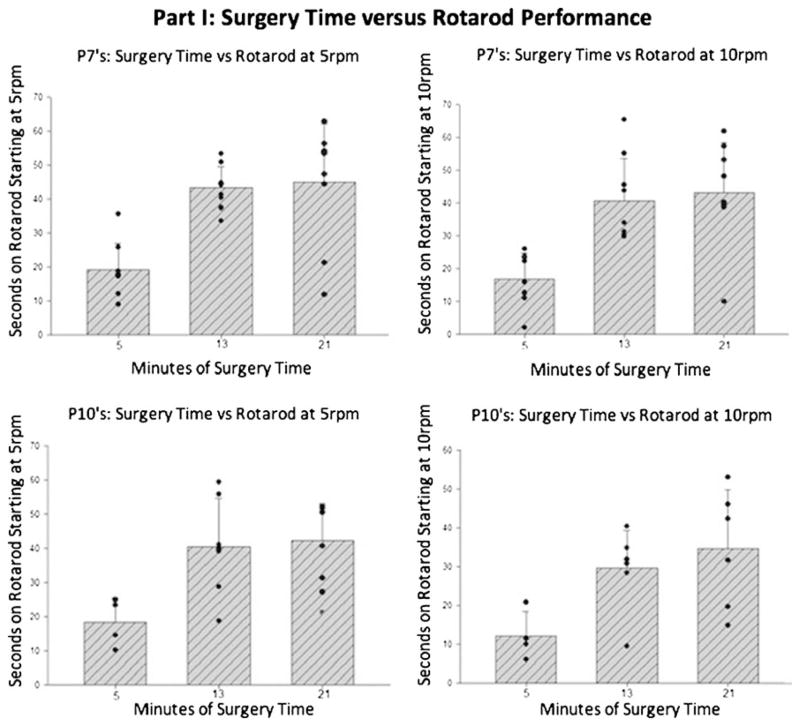
Surgery time versus neurobehavior: For long-term neurobehavior of 7-day-old rat pups, comparison of surgery time groups showed rotarod latency scores (Fig. 2) of the 5-min group to be significantly different from the 13 and 21 min groups, with no significant difference between the latter two groups (P<0.05). The same distribution of results was also seen for the 10-day-old rat pups (Fig. 2).
Fig. 1.
Part IA of the study examining the relationship between surgery time and infarction volume. These are graphs with representative photographs of hypoxic-ischemic neocortical infarction (light areas) after triphenyltetrazolium chloride staining at 48 h after hypoxic injury of 7- (P7) and 10-day-old (P10) rat pups. The surgery time was the time from skin incision to completion of ligation. There was a significant difference between the 5 min groups in comparison with the 13- or 21-min groups (P<0.05). There was no significant difference observed between the 13- and 21-min groups
Fig. 2.
Part IB of the study evaluating the relationship between surgery time and rotarod performance (reflected as latency time). These are representative graphs of how long it took the 7- and 10-day old rats to fall from the accelerating treadmill rotating at 5 or 10 rpm. At 5 rpm, there was significantly worsened performance for the 5-min group when compared with the 13- and 21-min groups (P<0.05). The same significant difference was also seen between the groups at higher speed of 10 rpm (P<0.05). The greatest brain injury correlated with the shortest exposure to isoflurane. The longest exposures to isoflurane showed the most protection of motor coordination and function
Part II
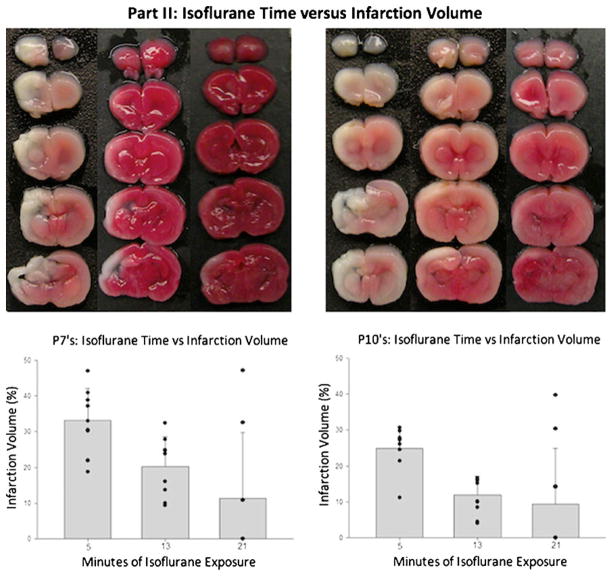
Isoflurane Time Versus Infarction Volume: In the second part of the study (Fig. 3), the surgery time (ITL) was fixed at 4 min, then isoflurane exposure continued until the end time-points of 5, 13, or 21 min. In the 7-day-old pups, largest infarction volume (mean 33.2%, SD 0.089) was seen in the 5-min exposure group with significantly reduced infarction observed in the 13-min (mean 20.3%, SD 0.082) and 21-min (mean 11.3%, SD 0.13) groups (P<0.05). For the post-natal day 10 pups (Fig. 3), the largest brain infarction volume (mean 24.8%, SD 0.062) was observed in the 5-min group and significantly smaller volumes of brain infarction were observed in the 13-min (mean 11.9%, SD 0.52) and 21-min (mean 9.3%, SD 0.15) groups (P<0.05).
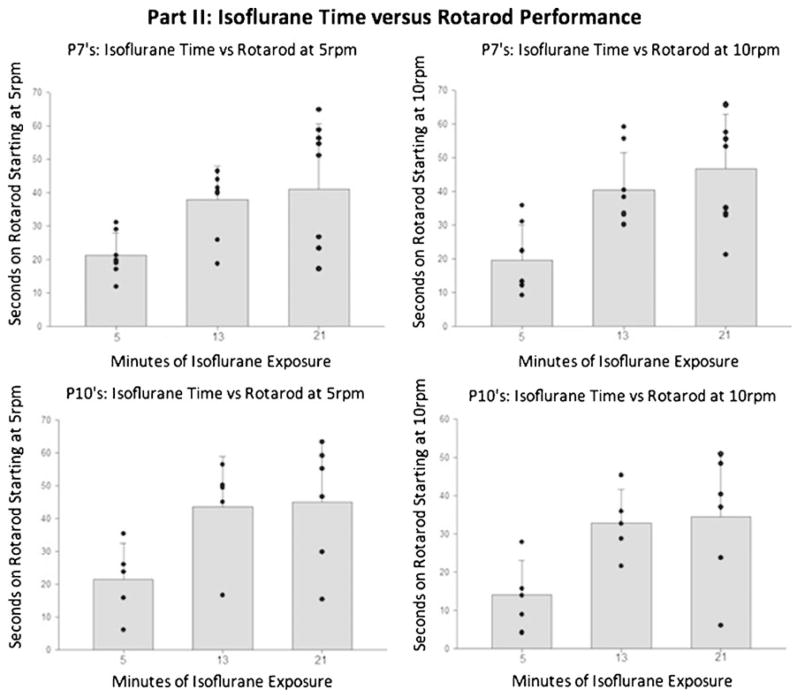
Isoflurane Time Versus Neurobehavior: A similar relationship can be seen when comparing the 4-min ITL groups with differing durations of exposure to isoflurane. For the 7-day-old pups (Fig. 4), the groups of shortest exposure to isoflurane demonstrated the worst performance on rotarod, and there was a distinct and significant difference when compared with the groups with longer exposures (13 and 21 min groups; P<0.05). Again, the same relationship was demonstrated in testing of the 10-day-old rat pups (Fig. 4). Consistently seen here is that the largest brain infarctions were observed in the 5-min surgery and 5-min isoflurane exposure groups, with a significant decrease in infarction size for the 13-min surgery and 13-min isoflurane groups. Also observed was a duration-correlated decrease of brain infarction observed in the 13- and 21-min groups (P<0.05). Greater standard deviation values were observed for the groups with extended isoflurane exposure, indicating a greater variability in the size of resulting infarction.
Fig. 3.
Part IIA of the study in which we showed the relationship between isoflurane time and infarction volume in 7- and 10-day-old rat pups. In this part of the study, there was a fixed surgery time of 4 min followed by continued isoflurane exposure to reach 5, 13, or 21 min. There were significantly increased infarction volumes for the 5-min surgery group in comparison with the extended surgery times of 13 and 21 min (P<0.05)
Fig. 4.
Part IIB of the study examining the relationship between isoflurane time and rotarod performance (measured as latency time). These are representative graphs of how long it took the 7- and 10-day old rats to fall from the accelerating treadmill rotating at 5 or 10 rpm. Part II of the study followed a fixed surgery time of 4 min followed by continued isoflurane exposure to reach 5, 13, or 21 min. At a starting speed of 5 rpm, there was significantly worsened performance for the 5-min group when compared with the 13- and 21-min group (P< 0.05). The differences were also consistently seen at the higher speed of 10 rpm between the 5 min compared with the 13- or 21-min group (P<0.05)
Discussion
We followed the model of severe and reproducible hypoxic-ischemic injury with homogeneous cortical infarction with most damage observed in the cortical regions of distribution of the middle cerebral artery. Earlier versions of this model were described by Rice et al., Ikonomidou et al. [13], Silverstein et al. and Schwartz et al. [12, 25, 29, 30]. Inconsistent infarction sizes were found to occur and a number of different explanations have been proposed for these observations. In particular, reasons for variations in the observed infarction size within the same strain and litter should be accounted for. Several explanations have been previously proposed by others, and in our investigation of this phenomenon, we have found isoflurane exposure to have the clearest relationship to infarction size.
One explanation offered was the effect of differing glucose levels. Hattori et al. demonstrated that blood sugar level differences were responsible for variation in the size of infarction. This may result from changes in feeding times, feeding amount, and temperature. Previous studies of glucose levels in hypothermic animals showed elevation in circulating glucose levels in the brain attributed to lessened cerebral glucose utilization and reduced insulin release. To address this, most research groups using this model currently follow the perioperative fasting period emphasized by Schwartz et al. for all experimental subjects to minimize the variation in glucose levels [29]. With this precautionary measure in place, variations in infarction size were still observed, prompting our investigation into other causes for the inconsistency.
Another proposed explanation was temperature variation. Fluctuations in ambient temperature are possible in the laboratory setting, away from normothermia (37°C), in the direction of moderate hypothermia (29°C), and profound hypothermia (21°C). Other effects of hypothermia to which neuroprotection has been attributed include inhibition of apoptosis, inhibition of glutamate release, and reduction of cerebral metabolism, with resultant preservation of high-energy phosphates, decreased intracellular acidosis, decreased lactic acid accumulation, preservation of endogenous antioxidants, reduced nitric oxide production, blockade of protein kinase inhibition, enhanced protein synthesis, reduced production of leukotrienes, blockade of brain edema formation, and blood–brain barrier disruption [6, 17, 27, 31, 36]. To control for the potential protective value of reduced temperatures, many groups described interoperative use of an incubator or heating pad to keep temperatures consistently elevated [6–8, 10, 17]. Professional laboratories maintain thermostatic control of ambient temperatures and minor fluctuations would not account for significant numbers of subjects with reduced infarction. Furthermore, temperature changes would not adequately account for differences in infarction seen within the same litter in which all subjects were positioned together.
Other metabolic, chemical, and physiologic factors influencing the severity of hypoxic-ischemic brain injury in young rat pups were postulated by others, including hyperglycemia, hypoglycemia, hypothermia, carbon dioxide exposure, ischemic preconditioning, drugs such as glucocorticoids, MK-801, calcium channel blockers, free radical inhibitors and scavengers, nitric oxide inhibitors, and nerve growth factor. Many of these conditions are difficult to control in this experimental model and have been postulated but not investigated. Appropriately controlled physiologic conditions of inbred animal subjects would account for the majority of those factors. Subtle fluctuations in blood glucose level would not account for major differences in infarction size after ligation and hypoxic exposure.
Another explanation was discussed by Dwyer et al. [7] who pointed out that possible variations in carotid blood flow following ligation could be responsible for differing degrees of perfusion and consequently, infarction size. Anatomically, inbred laboratory animal strains are bred and assessed for physiologic consistency by licensed laboratory animal suppliers, and while occasional differences in carotid anatomy and/or physiologic response are possible, they would not account for the significant numbers of subjects with reduced infarction. Further, surgical manipulation of the carotid is kept consistent across all subjects, and variations in infarction size corresponded with the duration of isoflurane exposure in situations of extended exposure.
Our study has demonstrated that isoflurane use during anesthesia provides a time-dependent neuroprotective effect, observed as a reduction in infarction volume with increased durations of exposure to isoflurane. Our findings are consistent with the existing body of research demonstrating the neuroprotective effects of isoflurane. Further, the mechanisms responsible for the observed neuroprotective effect of isoflurane have been shown to include inhibition of apoptosis, promotion of cellular proliferation, and enhancement of cellular survival, shown through in vitro and in vivo models [32, 38]. Even more pathways involved include the sphingosine-1-phosphate, phosphatidylinositol-3-kinase, and Akt pathways, as well as the nitric oxide synthase pathway [38, 42]. The reduction in infarction observed with increased exposure to isoflurane has also been consistent across the two different age groups of 7- and 10-day-old pups which may then account for infarction inconsistencies experienced by different groups using either neonatal or infant/adolescent age rat pups.
In considering other anesthetics for use instead of isoflurane, it is important for groups using this preclinical model to note evidence of each agent’s particular neuroprotective effects. Several other volatile inhalational and injectable intravenous anesthetics have been shown to provide neuroprotection against apoptosis and inflammation in ischemic brain injury, including desflurane, halothane, isoflurane, sevoflurane, nitric oxide, as well as barbiturates, propofol, and ketamine. Preconditioning mechanisms proposed to be responsible for the neuroprotective effects of anesthetics include Akt activation, nitric oxide pathways, ATP-sensitive potassium channels, cerebral blood flow changes, adenosine A1 receptor activation, and p38-mitogen-activated protein kinase-related pathways [3, 9, 19, 40, 43]. In this model of neonatal hypoxia-ischemia, inhalational anesthetics are most often chosen for their efficacy, safety in handling, and convenience; the most common ones being isoflurane, halothane, and sevoflurane. As the other anesthetics have not been evaluated in the current study, with regard to potential protective effects that may be exhibited, it is our suggestion that groups utilizing this model who are considering other anesthetics should evaluate their particular anesthetic for a similar phenomenon as observed here. Isoflurane preconditioning in particular has been demonstrated to reduce neonatal rat brain cell loss and injury [22]. Similarly, studies in transient and permanent focal stroke in rats used preconditioning with halothane which reduced infarction volume [15]. Another commonly use anesthetic in this model, sevoflurane, has been shown in preconditioning studies with global brain ischemia where it was found to reduce neuronal injury [24].
Conclusions
This study demonstrated that brain infarction inconsistencies in the neonatal hypoxia-ischemia rat pup model are related to the operation time. Decreased operation times and isoflurane exposure improve the consistency of brain infarction in the model. The observed time-dependent decrease in brain infarction is correlated with isoflurane exposure time. In this model, the intraoperative exposure to isoflurane, previously demonstrated as a neuroprotectant, may provide a notable neuroprotective conditioning effect against the ischemic injury. With use of inhalational anesthetics with neuroprotective properties such as isoflurane, maintaining a consistently shorter surgery time will lead to production of a more consistent infarction and model. Further, our results may place emphasis on the protective value of isoflurane, enhancing its regard for use in clinical settings where it would be of significant value [1, 25].
Contributor Information
Hank Chen, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA.
Michael Burris, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA.
Adrain Fajilan, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA.
Fred Spagnoli, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA.
Jiping Tang, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA.
John H. Zhang, Email: johnzhang3910@yahoo.com, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, CA 92350, USA. Departments of Neurosurgery, Loma Linda University, Loma Linda, CA 92350, USA
References
- 1.Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1(2):74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–8. doi: 10.1097/ALN.0b013e318194b5ff. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj A, Castro AF, Alkayed NJ, Hurn PD, Kirsch JR. Anesthetic choice of halothane versus propofol: impact on experimental perioperative stroke. Stroke. 2001;32:1920–5. doi: 10.1161/01.str.32.8.1920. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZY, Wang L, Asavaritkrai P, Noguchi CT. Up-regulation of erythropoietin receptor by nitric oxide mediates hypoxia preconditioning. J Neurosci Res. 2010;88(14):3180–8. doi: 10.1002/jnr.22473. [DOI] [PubMed] [Google Scholar]
- 5.Dalkara T, Yoshida T, Irikura K, Moskowitz MA. Dual role of nitric oxide in focal cerebral ischemia. Neuropharmacology. 1994;33 (11):1447–52. doi: 10.1016/0028-3908(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer BE, Nishimura RN, Fujikawa DG. Cerebral hypoxia-ischemia in immature rats: methodological considerations. Exp Neurol. 1988;99:772–7. doi: 10.1016/0014-4886(88)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Garcia J, Yoshida Y, Chen H, Li Y, Zhang Z, Lian J, et al. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Gidday JM, Shah AR, Maceren RG, Wang Q, Pelligrino DA, Holtzman DM, et al. Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxic preconditioning. J Cereb Blood Flow Metab. 1999;19(3):331–40. doi: 10.1097/00004647-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–96. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 11.Hattori H, Wasterlain CG. Posthypoxic glucose supplement reduces hypoxic-ischemic brain damage in the neonatal rat. Ann Neurol. 1990;28(2):122–8. doi: 10.1002/ana.410280203. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265(5180):1883–5. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidou C, Price MT, Mosinger JL, Friedrich G, Labruyere J, Salles KS, et al. Hypobaric-ischemic conditions produce glutamate-like cytopathology in infant rat brain. J Neurosci. 1989;9:1693–700. doi: 10.1523/JNEUROSCI.09-05-01693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston MV. new concepts in cerebral ischemia. Boca Raton FL: CRC Press; 2002. Neonatal hypoxic-ischemic brain insults and their mechanisms; pp. 31–61. [Google Scholar]
- 15.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke K, Isaev NK, et al. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 16.Kehl F, Payne RS, Roewer N, Schurr A. Sevoflurane-induced preconditioning of rat brain in vitro and the role of KATP channels. Brain Res. 2004;1021:76–81. doi: 10.1016/j.brainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–28. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laptook AR, Corbett RJ. The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol. 2002;29:623–49. doi: 10.1016/s0095-5108(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108(6):1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–13. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin T, He Y, Wu G, Khan M, Hsu C. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 22.McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesth Analg. 2007;104:1066–77. doi: 10.1213/01.ane.0000260321.62377.74. [DOI] [PubMed] [Google Scholar]
- 23.Nandagopal K, Dawson TM, Dawson VL. Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. J Pharmacol Exp Ther. 2001;297:474–8. [PubMed] [Google Scholar]
- 24.Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034:147–52. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Ratan RR. Beyond neuroprotection to brain repair: exploring the next frontier in clinical neuroscience to expand the therapeutic window for stroke. Transl Stroke Res. 2010;1(2):71–3. doi: 10.1007/s12975-010-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 27.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–5. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 28.Safar PJ, Kochanek PM. Therapeutic hypothermia after cardiac arrest. N Engl J Med. 2002;346:612–3. doi: 10.1056/NEJM200202213460811. [DOI] [PubMed] [Google Scholar]
- 29.Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–9. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz PH, Massarweh WF, Vinters HV, Wasterlain CG. A rat model of severe neonatal hypoxic-ischemic brain injury. Stroke. 1992;23(4):539–46. doi: 10.1161/01.str.23.4.539. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein F, Buchanan K, Johnston MV. Pathogenesis of hypoxic-ischemic brain injury in a perinatal rodent model. Neurosci Lett. 1984;49:271–7. doi: 10.1016/0304-3940(84)90301-x. [DOI] [PubMed] [Google Scholar]
- 32.Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev. 1997;19:326–38. doi: 10.1016/s0387-7604(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 33.Wise-Faberowski L, Raizada K, Sumners C. Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth Analg. 2001;93:1281–7. doi: 10.1097/00000539-200111000-00051. [DOI] [PubMed] [Google Scholar]
- 34.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–50. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neuroscience. 2008;154:1002–8. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakhary R, Miller JA, Miller FS. Hypothermia, asphyxia, and brain carbohydrates in newborn puppies. Biol Neonate. 1967;11:36–49. doi: 10.1159/000240053. [DOI] [PubMed] [Google Scholar]
- 38.Zhang RL, Chopp M, Chen H, Garcia JH. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2 H) middle cerebral artery occlusion in the rat. J Neurol Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–702. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurological outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–70. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 41.Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- 42.Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol Pharmacol. 2004;65:1172–80. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41(7):1521–7. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]