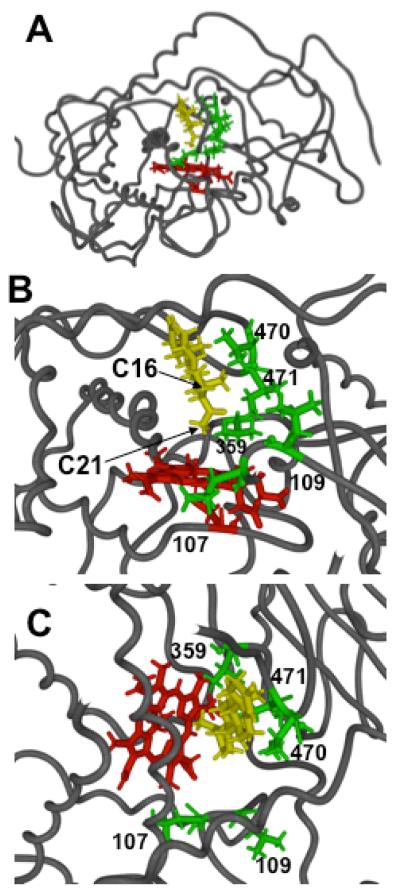
Figure 2.
Computer model of CYP21A2 with heme and ferryl oxene in red, docked P4 in yellow, and sidechains of the labeled residues studied here in green. Panel A, entire structure showing backbone atoms of protein, looking down axis of I-helix, plus all atoms of heme, P4, and residues L107, L109, V359, V470, and I471. Panels B and C, expanded views of active site with P4 hydrogen atoms at C-21 and C-16 labeled as viewed from side (Panel B) or top (Panel C). Distances in Table 1 are derived from the last 40 ps of a 100 ps molecular dynamics simulation with P4 docked in the orientation shown.