Figure 4.
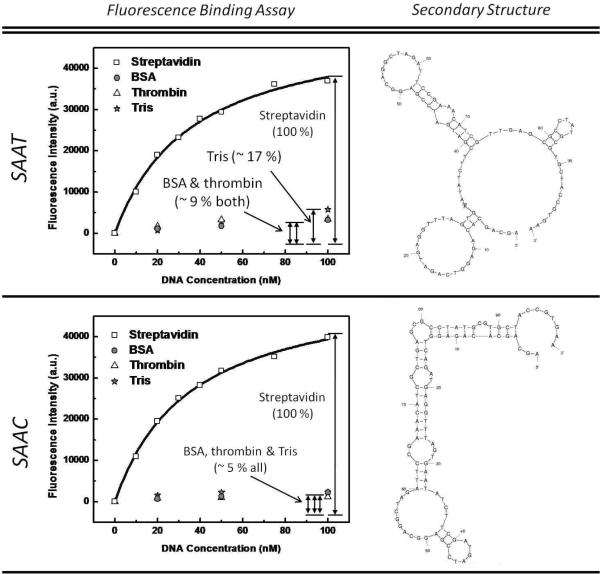
Characterization of SA aptamer sequences differing by a single nucleotide. The two consensus sequences showed similar affinities: Kd SAAC=35.2 ± 2.4 nM and Kd SAAT =36.2 ± 3.6 nM. However, the two sequences have distinct secondary structures and exhibit notable differences in specificity for BSA, thrombin and Tris. In comparison to the SAAT sequence, the SAAC sequence showed ~ 2-fold lower binding to BSA and thrombin, and ~ 3-fold lower binding to Tris coated beads.